Abstract
Neurodevelopmental disorders, including autism spectrum disorders, are highly male biased, but the underpinnings of this are unknown. Striatal dysfunction has been strongly implicated in the pathophysiology of neurodevelopmental disorders, raising the question of whether there are sex differences in how the striatum is impacted by genetic risk factors linked to neurodevelopmental disorders. Here we report male-specific deficits in striatal function important to reward learning in a mouse model of 16p11.2 hemideletion, a genetic mutation that is strongly associated with the risk of neurodevelopmental disorders, particularly autism and attention-deficit hyperactivity disorder. We find that male, but not female, 16p11.2 deletion animals show impairments in reward-directed learning and maintaining motivation to work for rewards. Male, but not female, deletion animals overexpress mRNA for dopamine receptor 2 and adenosine receptor 2a in the striatum, markers of medium spiny neurons signaling via the indirect pathway, associated with behavioral inhibition. Both sexes show a 50% reduction of mRNA levels of the genes located within the 16p11.2 region in the striatum, including the kinase extracellular-signal related kinase 1 (ERK1). However, hemideletion males show increased activation in the striatum for ERK1, both at baseline and in response to sucrose, a signaling change associated with decreased striatal plasticity. This increase in ERK1 phosphorylation is coupled with a decrease in the abundance of the ERK phosphatase striatum-enriched protein-tyrosine phosphatase in hemideletion males. In contrast, females do not show activation of ERK1 in response to sucrose, but notably hemideletion females show elevated protein levels for ERK1 as well as the related kinase ERK2 over what would be predicted by mRNA levels. These data indicate profound sex differences in the impact of a genetic lesion linked with neurodevelopmental disorders, including mechanisms of male-specific vulnerability and female-specific resilience impacting intracellular signaling in the brain.
Similar content being viewed by others
Introduction
Neurodevelopmental disorders, including autism spectrum disorders (ASD) and attention-deficit hyperactivity disorder (ADHD), are highly male biased. For example, ASD are strongly sex biased, occurring in four males for every one female,1 rising as high as seven males for every one female in cases with normal IQ.2 However, the mechanisms leading to sex differences in prevalence are unknown. A number of theories have been developed that suggest that sex differences in neural function contribute to risk of neurodevelopmental disorders.3, 4 Human genetic studies indicate that the male bias in autism has its foundation in differential vulnerability to genetic lesions. Compared with males, females require a higher burden of genetic mutations and copy number variations (CNVs) to be diagnosed with autism.5, 6 Indeed, even highly penetrant ASD-linked CNVs predominantly affect males,7 suggesting that the female brain may be resilient in response to genetic lesions that are strongly associated with diagnosis in males.
Animal models of genetic mutations and CNVs associated with neurodevelopmental disorder have the potential to provide significant insights into the molecular pathophysiology of the male bias in diagnoses. A CNV on chromosome 16 resulting in the loss of one copy of the 16p11.2 region profoundly increases the risk of diagnosis of neurodevelopmental disorders, particularly autism8 and ADHD,9 and even 16p11.2 heterozygous deletion carriers that do not meet specific diagnostic criteria often display language deficits and autism-like symptoms.9, 10 However, the risk of a psychiatric diagnosis is elevated in male 16p11.2 heterozygous deletion carriers over female carriers.9, 11 Human 16p11.2 deficiency syndrome can be very closely modeled in mice via hemideletion of chromosome 7qF3,12 as the genetic architecture of this region is highly conserved. In both species, this region contains 27 genes, including the gene encoding extracellular-signal related kinase 1 or ERK1 (the gene is also known as mitogen-activated protein kinase 3 (mapk3)).13, 14 ERK1 and the closely related kinase ERK2 have a crucial role in neuronal function and memory formation.15, 16, 17 However, prior studies describing the effects of 16p11.2 hemideletion in animal models have not examined whether the impact of the CNV differs between males and females,12, 18, 19, 20 despite evidence for sex differences in ERK1 and ERK2 function.21
Disruptions in striatal function have been implicated in many neurodevelopmental disorders. The salience of rewards and the ability to associate actions with rewarding outcomes22, 23 are mediated through striatal signaling and are thought to underlie many clinical symptoms of neurodevelopmental disorders,24, 25, 26 including the symptoms of autism and ADHD. Structural abnormalities of the striatum have been consistently seen in neurodevelopmental disorders,27, 28, 29, 30, 31, 32 including 16p11.2 hemideletion specifically,33 and these abnormalities have been linked with the severity of repetitive symptoms and errors in problem solving.28, 34 Functional deficits in striatal activity are seen in individuals with autism in response to both social and nonsocial rewards.31, 34, 35, 36 Interestingly, activation of the striatum in response to rewards differs between men and women,37 but sex differences in the underlying molecular processes supporting reward responses in this region are not well understood. The significance of the striatum to mediating these cognitive domains raises the question of whether this region is differentially impacted by genetic risk factors linked with neurodevelopmental disorders in a sex-dependent manner. Here we report male-specific deficits in goal-directed learning and motivation in a mouse model of 16p11.2 hemideletion,12 linked with sex-specific alterations in ERK1 signaling in the striatum.
Materials and methods
Animals
All animals were cared for in accordance with the guidelines of the National Institutes of Health and were approved by the University of Pennsylvania and Washington State University Institutional Animal Care and Use Committees. Colony founders from the 16p11.2 hemideletion (del/+) line generated in the laboratory of Dr Alea Mills were obtained from Jackson Laboratories (Bar Harbor, ME, USA; male founders: B6129S-Del(7Slx1b-Sept1)4Aam/J; Jackson Laboratories Stock no. 013128; female founders: Females; B6129SF1/J; Jackson Laboratories Stock no.101043). Male and female animals aged >70 days were used in all experiments. Additional methodological details can be found in Supplemental Information.
Behavioral testing
Operant testing
Animals engaging in operant testing were moved to a 0900–2100 hours reversed light cycle to permit testing during the dark period. Food restriction was used to maintain animals at 85–95% of free feeding weight for the duration of testing. Testing of fixed ratio (FR), progressive ratio and five-choice serial reaction time schedules were conducted exactly as previously described.38
Sucrose preference
Animals were singly housed prior to sucrose testing. Sucrose solution (4%) was prepared in deionized water and provided in the home cage using water bottles identical to those that provided standard water, as previously described.39 Animals were allowed to freely choose between the bottles containing water or sucrose for 72 h.
Auditory brainstem response
Hearing ability of 16p11.2 +/− mice and wild-type (WT) littermates was tested by recording anesthetized auditory brainstem responses as previously described.40
RNA quantification
Mice were killed at the onset of the dark period. Coronal sections through the striatum, including dorsomedial and dorsolateral striatum, nucleus accumbens and ventral pallidum were removed on ice using a mouse brain matrix allowing 1 mm sections, and tissue was preserved with RNAlater. RNA was extracted using Trizol (Ambion, Carlsbad, CA, USA) and converted to cDNA. Gene expression for the genes in the 16p11.2 region was assessed using Taqman assays directed at the appropriate targets, analyzed via high-throughput gene expression as measured by a Fluidigm Biomark HD 96 × 96 array and/or a Viia7 Real Time PCR system (Life Technologies, Carlsbad, CA, USA). Expression normalized to the geometric mean of glyceraldehyde 3-phosphate dehydrogenase (gapdh) and hprt was calculated using the comparative Ct method. Data were analyzed as t-tests within sexes using GraphPad Prism 6.0 (San Diego, CA, USA).
Protein quantification
Mice were killed at the onset of the dark period. For experiments comparing sucrose consumption to baseline (Figures 4 and 5), 4% sucrose was provided to animals via an additional bottle placed next to the home cage water bottle. All animals had undergone previous sucrose preference testing to remove novelty-induced aversion, and animals were observed to ensure sucrose was consumed. Animals were killed under either basal conditions or 40 min after the start of sucrose consumption. Brains were rapidly removed and coronal sections through the striatum were removed on ice in a similar manner as sections used for RNA analysis. Tissue was flash-frozen for later western blotting analysis and kept at −80 °C. Proteins were detected using standard western blotting techniques, using fluorescent secondary antibodies (Licor, Lincoln, NE, USA) enabling simultaneous detection of total and phosphorylated proteins. Data were analyzed with GraphPad Prism 6.0 using two-way analyses of variance, with Tukey multiple comparison tests for post hoc analysis and t-tests for direct comparisons.
Results
Action–outcome associations and motivation are impaired in male, but not in female, del/+ mice
Chr7qF3-deficient (del/+) and WT male and female mice were trained on a fixed-ratio 1 (continuous reinforcement) nosepoke task in a nine-hole mouse operant chamber (Figure 1a) while under mild food restriction, which had similar effects on body weight regardless of sex or genotype (Supplementary Figure 1). WT animals of both sexes rapidly learned to associate a nosepoke action to the center hole with the delivery of sweetened reinforcement at the magazine within 2–3 days of training. Male del/+ animals were impaired in acquiring this relationship (Figure 1a), requiring 7–8 days of training on average to reach levels of responding comparable to WT males. In contrast, female del/+ mice were unimpaired in learning this basic association in comparison to WT females. To understand the behavioral patterns contributing to deficits in FR1 learning in del/+ males, we examined the distribution of nonreinforced responses to the holes flanking the target hole on the first day of FR training in our first cohort (Figure 1b). In the early stages of training, it would not be clear to an animal which of the various actions it performed was the trigger for reward delivery, only that it had performed actions in the vicinity of the center hole immediately prior to reinforcement. Therefore, animals that are in the process of making an association between their actions and the rewarding outcome would be expected to make more response attempts at holes near the center of the array that are more likely to have been possible triggers for reward delivery and fewer at the holes at the edge of the array. WT males, and all females regardless of genotype, performed the majority of their nonreinforced responses to the two holes flanking the reinforced center hole. Del/+ males, in contrast, showed a more general pattern of responding and did not focus their responses to the area near the target hole in the early stages of training (Figure 1b), indicating that they were impaired in directing their actions to earn reinforcement. We also examined the rate of nonreinforced responses and found that these were uniquely reduced in del/+ males, despite displaying normal levels of activity in the operant chamber during FR1 training (Supplementary Figure 2). Thus del/+ males show deficits in performing the specific exploratory behaviors WT males employ in an attempt to elicit more frequent reinforcement, despite not being different in activity and exploration of the chamber in general. To determine whether del/+ males showed more general learning deficits, we examined behavior in two Pavlovian tasks, a Pavlovian cued approach task assessed prior to FR1 training (Supplementary Figure 3) and a Pavlovian contextual fear conditioning task (Supplementary Figure 4). We found that del/+ males displayed normal learning and performance in both Pavlovian tasks, indicating that their deficits appeared limited to learning operant action–outcome associations.
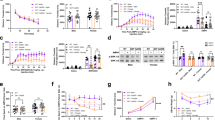
A mouse model of 16p11.2 hemideletion shows male-specific impairments in operant learning and motivation. (a) Male chr7qF3 deficient12 (16p11 del/+) males were significantly delayed at acquiring a nosepoke response under a fixed ratio 1 schedule of reinforcement compared with wild-type males (genotype × time interaction F(7,420)=2.1, P=0.05). In contrast, female 16p11 del/+ animals learned at a rate indistinguishable from female wild type (no effect of genotype or genotype × day interaction). No main effects of sex were seen in an analysis of variance (ANOVA) of all four groups or male and female wild type alone. (b) During shaping of the operant response, nonreinforced responses in the nine-hole nosepoke array were preferentially made at the holes flanking the reinforced hole by wild-type males but not by del/+ males (χ2 test comparing del/+ response distribution to wild-type response distribution, χ2(3)=43.7, P<0.0001). In contrast, both wild-type and del/+ females made the majority of their nonreinforced responses to the target-proximal holes (χ2 not significant). (c) In a progressive ratio test of motivation, del/+ males ceased responding after significantly fewer trials than wild-type males (t(56)=2.91, P=0.005) while del/+ females were no different than wild type (t-test not significant). No main effects of sex were seen in ANOVA comparing all four groups or in t-tests comparing male and female wild-type mice. (d) Del/+ animals of either sex showed no difference in sucrose preference (unpaired t-tests not significant). n in all figures=7–10 per group. Panels (a, c and d) depict mean±s.e.m. Panel (b) depicts mean proportions. *Indicates significant difference from wildtype of the same sex. §Indicates a significant gene × day of testing interaction.
Difficulties in acquiring operant responding may be driven by deficits in motivation to work for reinforcement. Following FR training, motivation was assessed in all groups via a progressive ratio schedule. Male del/+ mice displayed diminished motivation to work for reinforcement compared with WT males, while female del/+ were unaffected (Figure 1c). Because the animals in operant testing are working for sweetened liquid, we assessed preference for free sucrose in the home cage to ensure that this did not contribute to behavioral deficits. Both male and female del/+ mice displayed equal preference for sucrose compared with WT (Figure 1d), consistent with a substantial literature dissociating preference from motivation in striatal circuits.41 Although this literature has typically described intact motivation for a reward despite a deficit in preference in addiction models, the current data indicate that the reverse scenario, deficits in motivation despite intact preference, can also occur.
A cohort of animals trained in operant testing continued on into the five-choice serial reaction time test (5-CSRTT),42 which distinguishes errors of response accuracy, impulsivity and inattention. For this task, animals were first trained that responses at any odd-numbered hole, not merely hole 5, would now be reinforced. Interestingly, while del/+ males made a similar number of reinforced responses as WT males, reinforced responses made by del/+ males were disproportionately targeted to hole 5 over the other possible reinforcing responses for the first several days (Supplementary Figure 5). This difficulty in acquiring a greater variety of reinforced responses suggests that del/+ males may express deficits in forming action–outcome associations because of interference from alternative behaviors. Once the responses to all odd-numbered holes were learned, animals were transitioned to the 5-CSRTT. Male del/+ animals performed fewer correct trials in the 5-CSRTT than controls, while no significant differences in performance were seen in the females (Figure 2). Decreased correct performance in male del/+ was associated with significantly increased numbers of incorrect trials, indicating reduced response accuracy but not errors indicative of impulsivity or inattention. This pattern of deficits is associated with striatal lesions that leave the cortex spared,43 a manipulation that reduces the ability of the animal to associate specific responses with reinforcement.44 Significantly, with additional training the incorrect response deficit in del/+ males was eliminated (Supplementary Figure 6), consistent with the idea that this deficit reflected an inability to acquire a new action–outcome association, rather than being driven by enduring attention deficits. Thus del/+ males appear to show a profound inability to correctly associate responses with rewarding outcomes. Deficits in response accuracy without significant increases in premature or omitted trials can be caused by striatal dysfunction leading to deficits in integrating cortical inputs.43, 44
Del/+ males demonstrate impairments in response accuracy in the five-choice serial reaction time task (5-CSRTT). (a) Schematic of the 5-CSRTT task. (b) Performance of wild-type and del/+ male animals on the 5-CSRTT. Del/+ males make significantly fewer correct trials (t(14)=2.01, P=0.03). However, investigation of different error types reveals that only incorrect trials were significantly increased in rate in del/+ males (t(14)=2.06, P=0.029), while premature and omitted trials were not significantly altered. As noted in the text, a deficit in response accuracy without evidence for deficits in impulse control, attention or visual acuity is most consistent with a striatal deficit mediating errors informing action–outcome associations. (c) Performance of wild-type and del/+ females on the 5-CSRTT. No significant difference in any response measure was detectable in del/+ females. Panels (b and c) depict mean±s.e.m. n=7–10 per group. *Indicates significant difference from wild type from the same sex.
Impairments in striatal function have been linked with inner ear dysfunction.45 Another mouse model of 16p11.2 hemideletion expressed on a pure C57BL6/J background displayed profound deafness.18 Therefore, we examined auditory brainstem responses in the current model to test for any hearing impairments that might confound our findings of striatal dysfunction. Del/+ animals in our model displayed no hearing impairments compared with WT mice (Supplementary Figure 7), indicating that hearing loss is not a typical consequence of 16p11.2 hemideletion in mice but is specific to the line on the Bl6/J background.
Gene expression for receptors specific to dopamine indirect pathway signaling in the striatum are elevated only in male del/+
The striatum is essential for the learning and execution of motivated behaviors and is substantially more impacted by hemideletion of 16p11.2 than other brain regions, such as the hippocampus.46 We next examined whether the loss of one copy of the 16p11.2 region led to differential patterns of gene expression in the striatum of male and female del/+ animals. Using a high-throughput quantitative reverse transcriptase-PCR array, we first assayed the expression levels of the 27 genes located in the mouse homolog of the 16p11.2 region on chromosome 7. We found that nearly all of these genes are expressed in the striatum, and there were no sex differences in the mRNA levels of any of the hemideleted genes in the striatum, which were all reduced by half in male and female del/+ mice (Supplementary Figures 8a and b). A notable gene located within the 16p11.2 region is the ubiquitous kinase ERK1 (mapk3). ERK1, and the closely related kinase ERK2 (mapk1), have been widely demonstrated to regulate neuronal function.13, 15, 16, 47 In the striatum, these kinases have been shown to have opposing roles, such that ERK1 diminishes striatal neural plasticity, whereas ERK2 enhances it.16, 48 As with the other genes in the 16p11.2 region, mRNA levels for ERK1 in del/+ animals were reduced by half, and the expression of ERK2 (mapk1), located outside the hemideleted region, was not altered in either sex by the hemideletion (Supplementary Figure 8c).
Striatal neuron populations are composed primarily of medium spiny neurons (MSNs), which can express either dopamine receptor 1 (D1 or Drd1) or dopamine receptor 2 (D2 or Drd2). The balance of activity between these neurons has a critical role in the acquisition of goal-directed behavior.49, 50, 51, 52 Activity at D1+ MSNs, forming the ‘direct pathway’, is necessary for the initiation of actions, while activity at D2+ MSNs, forming the ‘indirect pathway’, serves to inhibit actions, including operant responding. Balanced activity between D1 and D2 MSNs is necessary to prevent both excessive off-target behaviors and excessive behavioral inhibition.43, 49, 51, 52, 53 A different model of 16p11.2 hemideletion found elevations in D2+ neurons in the striatum on postnatal day 154 but did not investigate animals in adulthood or examine males and females separately. The current data are consistent with this prior finding and suggest that these effects may persist into adulthood and should be a target of future investigation. Because of the importance of these receptors to striatal circuits and the operant deficits we observed in del/+ males, we investigated the expression of these receptors in the adult striatum.
Male del/+ animals showed elevations in mRNA for D2, along with adenosine 2a receptor which is expressed specifically on D2-positive MSNs (Figure 3a). In contrast, female del/+ animals showed no change in the expression of either receptor (Figure 3b). The metabotropic glutamate receptor mGluR5 (Grm5), which is expressed on both D1 and D2 MSNs, and along with dopamine regulates striatal neuron signaling, was elevated in both male and female del/+. Thus, while both sexes are impacted at the level of gene expression in the striatum for both 16p11.2-specific genes and mGluR5, male del/+ animals display specific vulnerability toward overexpression of Drd2 and adenosine 2a receptor, which would be expected to drive deficits in motivation and action initiation.49, 52
Receptors specific to the striatal indirect pathway are only elevated in male animals with 16p11.2 hemideletion. (a) There were no changes in expression in dopamine receptor 1 (Drd1a) in del/+ males. However, the striatum of del/+ males shows significant elevations in dopamine receptor 2 (Drd2, t(16)=2.47, P=0.02), adenosine receptor 2a (Adora2a, t(14)=3.05, P=0.008) and metabotropic glutamate receptor 5 (mGluR5/Grm5, t(12)=2.29, P=0.04). (b) In female del/+, there were no changes in expression in any receptor except Grm5, which was elevated in expression compared with wild-type females (t(17)=2.83, P=0.01). n=6–10 per group. All panels depict mean±s.e.m. *Indicates significant difference from wild type from the same sex.
The male-specific deficits in operant behavior and striatal dopamine receptor expression induced by loss of one copy of the 16p11.2 region raised the question of whether there are differences in dopamine synthesis or turnover within corticostriatal circuits. There were no differences in total dopamine levels in either the dorsal or ventral striatum or in the prefrontal cortex as measured by HPLC, and the ratio of dopamine to its metabolites HVA and DOPAC were also unaltered (Supplementary Figure 9). Thus changes in midbrain dopamine neurons are unlikely to account for behavioral deficits and mRNA changes seen in del/+ males, raising the question of whether molecular abnormalities within the striatum drive vulnerability in these males.
16p11.2 hemideletion leads to male-specific abnormalities in ERK1 phosphorylation at baseline and in response to natural rewards and female-specific increases in ERK protein levels
The kinase ERK1 is located in the 16p11.2 region and is a central regulator of protein–protein interactions of genes within the region.55 Reward-directed behavior is profoundly influenced by ERK phosphorylation and ERK1 dosage in the striatum,15, 16 such that ERK1 signaling decreases neuronal activity and the ability to associate reward, while the activity of the close homolog ERK2 enhances these functions.16, 48 Phosphorylation of ERK proteins in the MSNs of the striatum occurs in response to a number of rewarding substances, including sucrose15, 47, 56, 57 and drugs of abuse.56, 58, 59, 60 Prior reports have indicated that, despite loss of one copy of ERK1, phosphorylation of ERK1 is elevated by 16p11.2 hemideletion in both mouse and human tissues,19, 61 but these studies did not examine sex differences.
Because the loss of one copy of ERK1 occurred in del/+ animals of both sexes but the behavioral impairments were specific to the males, we questioned whether there might be sex differences in the impact of 16p11.2 hemideletion on the activation of ERK intracellular signaling following the consumption of sucrose, the natural reward driving responding in our operant task. ERK proteins are known to be phosphorylated shortly after the initial exposure to a reward and during operant training.15, 47 To interrogate this signaling and how it might be impacted by 16p11.2 hemideletion, we provided animals with short-term access to sucrose in the home cage and examined ERK1 and ERK2 phosphorylation 40 min after sucrose consumption began, a time point where ERK striatal phosphorylation is increased following reward delivery58, 60, 62 and that mimics the unexpected sucrose delivery in the operant chamber during the early stages of goal-directed learning.
In del/+ males, ERK1 phosphorylation under basal conditions was slightly elevated (Figure 4a), consistent with prior literature.19 ERK1 phosphorylation was also elevated in response to sucrose consumption in both del/+ and WT males. However, the elevation of ERK1 phosphorylation in del/+ males far exceeded that of WT males, such that the ratio of phosphorylated ERK1 relative to the normalizing protein GAPDH was significantly elevated in del/+ males (Figure 4b). As before, ERK1 protein levels were reduced in del/+ males to 50% of WT male levels (Figure 4c). ERK2 phosphorylation in the striatum was not significantly affected by genotype or sucrose. Thus ERK1 in the striatum of del/+ males is hyperphosphorylated, and this condition is significantly exacerbated by exposure to sucrose, suggesting aberrant ERK1-mediated signaling events in the striatum of del/+ males in response to natural rewards.
Hemideleted males show excess extracellular-signal related kinase (ERK1) phosphorylation in the striatum in response to sucrose, a natural reward. Because ERK1 is located within the 16p11.2 region and its activity is detrimental to striatal function, we examined whether natural reward (sucrose) alters the activation of this protein, which might contribute to deficits in goal-directed learning and motivation. Adult male wild-type and del/+ males were singly housed and given access to sucrose for 40 min at the onset of the dark period (sucrose conditions) or were undisturbed (basal conditions). We examined ERK1 and ERK2 phosphorylation in the striatum at the end of this period. (a) Relative to the total amount of ERK1 protein, phosphorylated ERK1 was elevated in response to sucrose (main effect sucrose F(1,31)=4.1, P=0.05) and in del/+ males in both conditions (main effect genotype F(1,31)=5.8, P=0.02). (b) Levels of phosphorylated ERK1 normalized to the loading control protein glyceraldehyde 3-phosphate dehydrogenase (GAPDH) indicated a significant and abnormal increase in phospho-ERK1 in del/+ males in response to sucrose (genotype × sucrose interaction F(1,29=3.6, P=0.05). (c) Total ERK1 protein was reduced by 50% in del/+ males under both conditions (main effect genotype F(1,29)=44.5, P<0.0001). (d) In response to sucrose consumption, phosphorylated ERK2 relative to total ERK2 was not altered in either genotype. (e) Phosphorylated ERK2 levels normalized to GAPDH were not different between groups. (f) Total ERK2 levels were not different between groups. (g) Representative western blotting images. Western blotting images are displayed as a heat map, ranging from high protein levels (red) to low protein levels (blue). B indicates basal samples and S indicates samples from animals that consumed sucrose. n=7–10 per group. Panels (a–f) depict mean±s.e.m. *Indicates significant difference from wildtype of the same sucrose condition. #Indicates a main effect of sucrose. §Indicates a significant gene × sucrose interaction.
Phosphorylation of ERK proteins in the brain are regulated by the activity of the upstream kinase mitogen-activated ERK (MEK). We investigated the levels and activity of MEK2 in the striatum to determine whether the increase in ERK1 phosphorylation in male del/+ was due to changes in upstream signaling mechanisms. MEK2 levels were not affected by 16p11.2 hemideletion, and it was equally activated in WT and del/+ males in response to sucrose (Supplementary Figure 10), indicating upstream signaling mechanisms, such as those mediated by mGluR5 signaling, were not responsible for the hyperphosphorylation of ERK1 in male del/+.
Studies demonstrating the involvement of ERK phosphorylation in response to rewarding substances have largely been conducted in males. Because del/+ females were unaffected in goal-directed learning by hemideletion of ERK1, it is possible that ERK signaling mechanisms in the striatum are differentially impacted by natural rewards in females. We therefore examined ERK1 and ERK2 phosphorylation in female WT and del/+ following sucrose consumption, paralleling the experiments in males. When striatal ERK was examined in females 40 min after the initiation of sucrose consumption, we found no evidence of induced ERK1 or ERK2 phosphorylation in females of either genotype in response to sucrose (Figure 5). This is in contrast to the induction of ERK1 phosphorylation in males, suggesting that there are sex differences in molecular signaling in response to natural rewards in the striatum. Unexpectedly, female del/+ had higher ERK1 and ERK2 protein levels than would be expected by gene expression levels alone. ERK1 protein levels reached approximately 75% of female WT levels, appreciably higher than the 50% of male WT ERK1 protein seen in male del/+ striatum (Figure 4). Likewise, total ERK2 protein levels were significantly elevated in del/+ females compared with WT females, despite similar levels of ERK2 mRNA expression in these groups (Supplementary Figure 8). Thus two significant sex differences in striatal ERK signaling emerge—first, the intracellular signaling initiated in response to natural rewards in males is not recapitulated in females, and second, the protein levels of ERK1 and ERK2 in the striatum appear to be significantly elevated in del/+ females beyond what would be expected based on genotype or mRNA levels.
16p11.2 hemideleted females show no changes in extracellular-signal related kinase (ERK1) phosphorylation in response to sucrose but show increased ERK1 and ERK2 protein levels. Because consumption of sucrose significantly increased ERK1 phosphorylation in male striatum, especially in del/+ males, we asked whether similar changes in ERK phosphorylation occur in the female striatum in response to unexpected sucrose delivery. Adult female wild-type and del/+ animals were singly housed and given access to sucrose for 40 min at the onset of the dark period (sucrose conditions) or were undisturbed (basal conditions). We examined ERK1 and ERK2 phosphorylation in the striatum at the end of this period. (a) Relative to the total amount of ERK1 protein, there were no changes in ERK1 phosphorylation in response to sucrose in females. (b) Phosphorylated ERK1 normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was not altered by sucrose but was significantly decreased in del/+ females (main effect genotype F(1,20)=5.48, P=0.02). (c) Total ERK1 in del/+ females was significantly reduced compared with wild-type females (main effect genotype F(1,18)=11.5, P=0.003). However, del/+ female ERK1 levels were approximately 75% of female wild-type levels, much greater than the 50% ERK1 protein levels seen in del/+ males compared with wild-type males. (d) Relative to the total amount of ERK2 protein, there were no changes in ERK2 phosphorylation in response to sucrose in females. (e) Phosphorylated ERK2 normalized to GAPDH was not altered by sucrose consumption but tended to be elevated overall in del/+ females (main effect genotype F(1,13)=2.9, P=0.11). (f) Total ERK2 levels were significantly increased in del/+ females compared with wild-type females (main effect genotype F(1,14)=5.8, P=0.03), paralleling the increases in expected levels of ERK1 protein in del/+ females. (g) Representative western blotting images. Western blotting images are displayed as a heat map, ranging from high protein levels (red) to low protein levels (blue). B indicates basal samples, S indicates samples from animals that consumed sucrose and L indicates a ladder lane. n=6–7 per group. Panels (a–f) depict mean±s.e.m. *Indicates significant difference from female wild type.
The apparent elevation in ERK1 protein levels in female del/+ animals led us to question whether intrinsic sex differences in ERK1 levels could account for this difference. Accordingly, we collected striatum from male and female WT and del/+ animals in a single experiment and analyzed ERK1 and ERK2 phosphorylated and total protein levels to allow us to directly compare protein levels across all conditions (Figure 6). ERK1 total protein levels were again reduced by 50% in del/+ males compared with WT males, but intriguingly ERK1 total protein was also reduced in the WT female striatum compared with levels seen in WT males (Figure 6a). Although the overall amount of ERK1 protein did not differ between del/+ males and del/+ females, when examined proportional to WTs of the same sex, female del/+ show an increased abundance of ERK1 protein. Because ERK1 mRNA levels were reduced to 50% of WTs in del/+ in both sexes, these data indicate that mechanisms regulating ERK protein abundance are enhanced in del/+ females. Indeed, this was seen not only in ERK1 but also in ERK2. Total ERK2 levels did not differ by sex but were significantly elevated solely in del/+ females compared with WT females (Figure 6), replicating our prior finding (Figure 5).
Elevated extracellular-signal related kinase (ERK1) phosphorylation is associated with decreased protein levels of striatal-enhanced phosphatase (StEP) in del/+ males. By analyzing male and female wild-type and del/+ striatal tissue in the same experiment, we were able to assess which, if any, sex-specific effects of 16p11.2 hemideletion on ERK1 and ERK2 activation and protein levels were due to intrinsic sex differences. (a) Total ERK1 protein was reduced in both male and female del/+ to 50% of male wild-type levels (main effect genotype F(1,27)=54.2, P<0.0001), but ERK1 protein was also significantly lower in female wild types than male wild types (main effect sex F(1,27=6.9, P=0.01), indicating a partial normalization of del/+ female ERK1 levels. (b) Consistent with the overall hemideletion of ERK1, 16p11.2 del/+ of both sexes have significantly decreased phosphorylated ERK1 normalized to the loading control beta-tubulin (main effect genotype F(1,30)=5.8, P=0.02), consistent with the reduction in ERK1 gene expression. (c) Male 16p11.2 del/+ show hyperactivated ERK1. Relative to the total amount of ERK1 protein available in each condition, del/+ males have significantly increased levels of phosphorylated ERK1 (male wt vs del/+ t(15)=2.1, P=0.05). Female del/+ do not show any increases in ERK1 phosphorylation relative to female wild types. (d) Total ERK2 protein was not altered in male del/+ but was significantly increased in female del/+ compared with female wild type (t(15)=2.5, P=0.02). (e) Relative to the loading control protein β-tubulin, del/+ females have significantly increased levels of pERK2 (genotype × sex interaction F(1,26)=4.6, P=0.04). (f) Relative to the total amount of ERK2, there were no differences in the amount of phosphorylated ERK2 caused by del/+ in either sex. There was a trend toward an overall increase in females (main effect sex F(1,23)=2.8, P=0.1). (g) Representative western blotting images depicting phosphorylated ERK1 and ERK2, total ERK1 and ERK2 and β-tubulin as a loading control in wild-type and del/+ male and female striatum. (h) Protein levels of the ERK-regulating phosphatase StEP 61 are significantly reduced in the striatum of del/+ males but not of del/+ females (sex × genotype interaction F(1,28)=4.4, P=0.04). (i) Representative western blotting images depicting total levels of StEP 61 and and β-tubulin as a loading control in wild-type and del/+ male and female striatum. Panels (a–f and h) depict mean±s.e.m. Western blotting images in panels (g and i) are displayed as a heat map, ranging from high protein levels (red) to low protein levels (blue). n per group=7–10. All samples were normalized to wild-type males to examine sex differences. *Indicates significantly different from wild type of the same sex. #Indicates a main effect of sex. §Indicates a significant gene × sex interaction.
We next asked how sex interacted with genotype to influence the previously observed differences in basal ERK phosphorylation. Despite the reduction of total ERK1 in del/+ males, they again showed a disproportionate level of phosphorylated ERK1, leading to elevations in the phospho-ERK1: total ERK1 ratio solely in del/+ males (Figure 6c), consistent with our prior experiment (Figure 4). In contrast, no enhancements in ERK1 phosphorylation normalized to total ERK1 levels were seen in female del/+. As in our previous experiments, there were no differences in ERK2 phosphorylation between WT and del/+ males. We observed elevated levels of phosphorylated ERK2 in del/+ females when normalized to the loading control, but this difference was no longer observed when normalized to total ERK2 levels, suggesting that this difference was driven by an increase in the overall abundance of the protein.
The persistent increase in phosphorylated ERK1 in del/+ males despite a lack of difference in the ERK kinase MEK (Supplementary Figure 10) led us to question whether mechanisms of dephosphorylation may be disrupted in the del/+ male striatum. We interrogated levels of the phosphatase striatum-enriched protein-tyrosine phosphatase (StEP).63, 64, 65 As its name indicates, StEP is highly enriched in the striatum as well as other neural tissues, and it is a critical negative regulator of ERK phosphorylation via neuronal excitability.66, 67 We found that total levels of one isoform, StEP61, were reduced specifically in male del/+ striatum (Figure 6h), suggesting that elevated phosphorylation of ERK1 in del/+ males may be driven by a decreased ability to negatively regulate phosphorylation. The relative phosphorylation of StEP61 (associated with inactivation of StEP) was not altered by del/+ in either sex (Supplementary Figure 11). Thus the behaviorally vulnerable del/+ males have profound disruptions in striatal molecular signaling, including abnormal ERK1 activation coupled with decreased levels of a key negative regulator of ERK phosphorylation. In contrast, the behaviorally unimpaired del/+ females show not only normalized basal ERK1 phosphorylation and normal StEP protein levels in the striatum but intriguing increases in ERK1 and ERK2 protein levels that may contribute to their resilience.
Discussion
Animal models of CNVs associated with neurodevelopmental disorders have the potential to provide mechanistic insights into the pathophysiology of the male bias in these disorders. In a mouse model of 16p11.2 hemideletion, directly modeling a human CNV strongly associated with autism and ADHD, we believe we provide the first demonstration of male-specific vulnerability to behavioral and molecular deficits caused by a genetic lesion. Male del/+ mice showed unique impairments in reward learning and motivation, coupled with excess phosphorylation of the kinase ERK1 at baseline and after consumption of sucrose, a natural reward. Female carriers of the deletion were resistant to these behavioral deficits and did not show increased ERK1 activation, instead showing elevated ERK1 and ERK2 protein levels. In addition, male del/+ mice displayed reductions in StEP, a negative regulator of ERK phosphorylation that may contribute to behavioral dysfunction in 16p11.2 del/+ males. Because ERK1 signaling has been shown to inhibit striatal function, whereas ERK2 signaling enhances it, it seems likely that the male-specific increase in striatal ERK1 phosphorylation may drive deficits in operant learning. In contrast, at least four mechanisms in female del/+ animals we observed may contribute to their resilience to behavioral deficits, two ways in which overall protein levels may be significant and two ways in which phosphorylation may be significant. First, total ERK1 protein levels in the female WT striatum are lower than is typical for male WTs, which may mean the reduction in total ERK1 in female del/+ has less of a deleterious impact when assessed against what is typical in a female WT. Second, the fact that ERK1 and ERK2 levels were elevated in the del/+ female brain over what would be expected from mRNA measurements suggests that there may be sex-specific mechanisms critical to the regulation of protein levels that drive increased molecular resilience in the female brain. There are not well-described mechanisms by which protein abundance might be expected to differ between the sexes, though one Y-chromosome-specific gene, USP9Y, has specific roles in protein ubiquitination and degradation,68 suggesting one possible mechanism for sex-dependent regulation of protein levels. Turning our attention to the phosphorylation of ERK proteins, we must also consider a role for differential regulation of protein kinase cascades between the sexes. A third mechanism by which females may maintain normal behavioral performance is the lack of baseline hyperphosphorylation of ERK1 in female del/+, coupled with normal StEP expression, suggesting that ERK kinase cascades in striatal neurons may be differentially activated in females. Corroborating this point is a fourth possible contributing mechanism that the molecular response in the striatum to experiencing natural rewards such as sucrose is females was not engaged in the same way as it was for the males. The lack of ERK phosphorylation in females of either genotype in response to sucrose that was observed even in WT males suggests, at minimum, a sex difference in the time course or magnitude of the intracellular signaling mechanisms induced by sucrose consumption. In contrast to the lack of known mechanism driving sex differences in ERK protein abundance, there are existing reports of large sex differences in the intracellular molecular cascades for at least one G-protein-coupled receptor,69 which supports the potential for differential neuronal protein phosphorylation regulation between males and females that might contribute to male behavioral vulnerability. It remains to be seen which of these differences in the impact of 16p11.2 hemideletion is most meaningful to preventing the behavioral deficits seen in male del/+, but they provide overwhelming evidence that the female brain is resilient on a molecular level to a CNV associated with neurodevelopmental disorders.
The cognitive systems supporting reward learning, including outcome prediction and motivation, are essential to social behavior, behavioral flexibility and impulse control,26, 70 behavioral domains that are heavily impacted in neurodevelopmental disorders. Different subregions of the striatum are thought to support separate aspects of goal-directed learning. Although dorsal striatal regions support the ability to associate actions with outcomes and stimuli with responses,71 the nucleus accumbens is essential for coding the motivational or incentive properties of an outcome.72 Del/+ males showed impairments in both acquiring an action–outcome response and maintaining motivation to work for rewards, suggesting that signaling might be impaired broadly across striatal subcompartments. Approximately 95% of neurons across both striatal subregions are MSNs. Del/+ males also showed increases in striatal gene expression specific to the D2 dopamine receptor and A2a adenosine receptor, both expressed solely on indirect pathway MSNs that contribute to behavioral inhibition.43, 49, 51, 52 A number of other autism-linked genes, including neurexins, neuregulins and Shank3, are enriched in the striatum,73, 74, 75, 76 and loss-of-function mouse models of these genes produce dysregulated synaptic function in the striatum localized to a specific subset of MSNs.73, 74, 76 Intriguingly, loss of function of several genes linked to ASD have also been linked to ERK hyperactivation,77, 78, 79 suggesting that this signaling pathway within the neurons participating in striatal circuits may form a common deleterious mechanism in neurodevelopmental disorders. Indeed, FMR1 knockout mice show not only ERK hyperactivation but also similar operant deficits in acquiring new action–outcome associations in 5-CSRTT training,80 suggesting that difficulties in the ability to learn new relationships between specific behaviors and reward is a common cognitive phenotype in multiple mouse ASD models linked by ERK dysfunction. It is not possible to know from the current data whether increased ERK1 phosphorylation in del/+ males is specific to a particular population of MSNs, but our data indicate that neuronal signaling cascades are likely to account for del/+ male behavioral vulnerability. Although our findings overall lend substantial support to the idea that striatal dysfunction appears to be a common mechanism contributing to the increased male risk for neurodevelopmental disorders, it is important to note that the striatum is only one part of broader neural circuits regulating motivated behavior. Indeed, the development of cortical regions essential for regulating striatal function are significantly impacted in early life by 16p11.2 hemideletion,18, 19 suggesting that multiple components of reward circuitry in the brain may be disrupted. It may even be the case that molecular dysfunction in the striatum is driven by abnormal development of corticostriatal circuits, especially sex-dependent programming of these circuits.
We repeatedly observed that female del/+ animals did not display the same behavioral deficits as male del/+, a striking observation given the profound sex bias in the diagnosis and severity of neurodevelopmental disorders.5 In addition to normalized expression of markers of the striatal indirect pathway, female del/+ did not show the hyperphosphorylation of ERK1 seen in male del/+. Indeed, females did not show detectable changes in ERK phosphorylation in response to sucrose regardless of genotype, suggesting sex differences in the dynamics of striatal intracellular signaling that may leave females less vulnerable to striatal dysfunction driven by 16p11.2 hemideletion. The increased abundance of ERK1 and ERK2 proteins in the female del/+ striatum may serve to normalize the baseline phosphorylation of ERKs and thus their downstream function. Although mice with a complete deletion of ERK1 were previously shown to have normal ERK2 protein abundance in the striatum,16 this work was only conducted in male mice, leaving the questions of sex-specific compensation and the impact of heterozygous deletion of ERK1 unaddressed. An intriguing open question is whether female del/+ display differences in protein abundance in other important signaling molecules. Sex differences in the impact of a genetic lesion on protein abundance is consistent with the well-known links between mutations in genes regulating protein synthesis and the risk of neurodevelopmental disorders.81 Another open question revolves around the mechanisms driving the differential impact of 16p11.2 hemideletion on males and females. Although adult gonadal steroids can regulate ERK phosphorylation,82, 83, 84 the differences may also result from early-life masculinization or the gene dosage of sex chromosome-linked regulators of protein abundance and function.68, 85, 86 Future studies systematically exploring the basis of this sex difference will be needed to uncover where and how in development sex acts to influence the impact of this CNV. Because neurodevelopmental disorders driven by genetic factors other than 16p11.2 hemideletion are also strongly male biased, this work could have the potential to reveal more general mechanisms and therapeutic avenues.
References
Volkmar FR, Pauls D . Autism. Lancet 2003; 362: 1133–1141.
Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health 2007; 28: 235–258.
Baron-Cohen S, Knickmeyer RC, Belmonte MK . Sex differences in the brain: implications for explaining autism. Science 2005; 310: 819–823.
Lai M-C, Baron-Cohen S, Buxbaum JD . Understanding autism in the light of sex/gender. Mol Autism 2015; 6: 1–5.
Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A . Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci USA 2013; 110: 5258–5262.
Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, Bergmann S et al. A higher mutational burden in females supports a ‘female protective model’ in neurodevelopmental disorders. Am J Hum Genet 2014; 94: 415–425.
Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet 2014; 94: 677–694.
Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med 2008; 358: 667–675.
Hanson E, Bernier R, Porche K, Jackson FI, Goin-Kochel RP, Snyder LG et al. The cognitive and behavioral phenotype of the 16p11.2 deletion in a clinically ascertained population. Biol Psychiatry 2014; 177: 785–793.
Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnusdottir B, Morgen K, Arnarsdottir S et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature 2014; 505: 361–366.
Duyzend MH, Eichler EE . Genotype-first analysis of the 16p11.2 deletion defines a new type of ‘autism’. Biol Psychiatry 2015; 77: 769–771.
Horev G, Ellegood J, Lerch JP, Son Y-EE, Muthuswamy L, Vogel H et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc Natl Acad Sci USA 2011; 108: 17076–17081.
Pucilowska J, Puzerey PA, Karlo JC, Galán RF, Landreth GE . Disrupted ERK signaling during cortical development leads to abnormal progenitor proliferation, neuronal and network excitability and behavior, modeling human neuro-cardio-facial-cutaneous and related syndromes. J Neurosci 2012; 32: 8663–8677.
Levitt P, Campbell DB . The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest 2009; 119: 747–754.
Shiflett MW, Brown Ra, Balleine BW . Acquisition and performance of goal-directed instrumental actions depends on ERK signaling in distinct regions of dorsal striatum in rats. J Neurosci 2010; 30: 2951–2959.
Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 2002; 34: 807–820.
Samuels IS, Saitta SC, Landreth GE . MAP’ing CNS development and cognition: an ERKsome process. Neuron 2009; 61: 160–167.
Portmann T, Yang M, Mao R, Panagiotakos G, Ellegood J, Dolen G et al. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. Cell Rep 2014; 7: 1077–1092.
Pucilowska J, Vithayathil J, Tavares EJ, Kelly C, Karlo JC, Landreth GE . The 16p11.2 deletion mouse model of autism exhibits altered cortical progenitor proliferation and brain cytoarchitecture linked to the ERK MAPK pathway. J Neurosci 2015; 35: 3190–3200.
Yang M, Mahrt EJ, Lewis F, Foley G, Portmann T, Dolmetsch RE et al. 16p11.2 deletion syndrome mice display sensory and ultrasonic vocalization deficits during social interactions. Autism Res 2015; 8: 507–521.
Mizuno K, Giese KP . Towards a molecular understanding of sex differences in memory formation. Trends Neurosci 2010; 33: 285–291.
Pasupathy A, Miller EK . Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature 2005; 433: 873–876.
Balleine BW, Liljeholm M, Ostlund SB . The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res 2009; 199: 43–52.
Báez-Mendoza R, Schultz W . The role of the striatum in social behavior. Front Neurosci 2013; 7: 233.
Dölen G, Darvishzadeh A, Huang KW, Malenka RC . Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013; 501: 179–184.
Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux a L, Pantazis D et al. Autism as a disorder of prediction. Proc Natl Acad Sci 2014; 111: 15220–15225.
Di Martino A, Kelly C, Grzadzinski R, Zuo X-N, Mennes M, Mairena MA et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry 2011; 69: 847–856.
Langen M, Bos D, Noordermeer SDS, Nederveen H, van Engeland H, Durston S . Changes in the development of striatum are involved in repetitive behavior in autism. Biol Psychiatry 2014; 76: 405–411.
Langen M, Durston S, Staal WG, Palen SJMC, van Engeland H . Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry 2007; 62: 262–266.
Haznedar MM, Buchsbaum MS, Hazlett Ea, LiCalzi EM, Cartwright C, Hollander E . Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. Am J Psychiatry 2006; 163: 1252–1263.
Voelbel GT, Bates ME, Buckman JF, Pandina G, Hendren RL . Caudate nucleus volume and cognitive performance: are they related in childhood psychopathology? Biol Psychiatry 2006; 60: 942–950.
Nickl-Jockschat T, Habel U, Michel TM, Manning J, Laird AR, Fox PT et al. Brain structure anomalies in autism spectrum disorder—a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp 2012; 33: 1470–1489.
Maillard A, Ruef A, Pizzagalli F, Migliavacca E, Hippolyte L, Adaszewski S et al. The 16p11.2 locus modulates brain structures common to autism, schizophrenia and obesity. Mol Psychiatry 2015; 20: 140–147.
Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY . Reward processing in autism. Autism Res 2010; 3: 53–67.
Greene DJ, Colich N, Iacoboni M, Zaidel E, Bookheimer SY, Dapretto M . Atypical neural networks for social orienting in autism spectrum disorders. Neuroimage 2011; 56: 354–362.
Kohls G, Schulte-Rüther M, Nehrkorn B, Müller K, Fink GR, Kamp-Becker I et al. Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci 2013; 8: 565–572.
Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc Cogn Affect Neurosci 2009; 4: 158–165.
Grissom NM, Herdt CT, Desilets J, Lidsky-Everson J, Reyes TM . Dissociable deficits of executive function caused by gestational adversity are linked to specific transcriptional changes in the prefrontal cortex. Neuropsychopharmacology 2015; 40: 1353–1363.
Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM . Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 2010; 151: 4756–4764.
Portfors CV, Roberts PD . Mismatch of structural and functional tonotopy for natural sounds in the auditory midbrain. Neuroscience 2014; 258: 192–203.
Berridge KC, Robinson TE . What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 1998; 28: 309–369.
Bari A, Dalley JW, Robbins TW . The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 2008; 3: 759–767.
Nishizawa K, Fukabori R, Okada K, Kai N, Uchigashima M, Watanabe M et al. Striatal indirect pathway contributes to selection accuracy of learned motor actions. J Neurosci 2012; 32: 13421–13432.
Agnoli L, Mainolfi P, Invernizzi RW, Carli M . Dopamine D1-like and D2-like receptors in the dorsal striatum control different aspects of attentional performance in the five-choice serial reaction time task under a condition of increased activity of corticostriatal inputs. Neuropsychopharmacology 2013; 38: 701–714.
Antoine MW, Hübner Ca, Arezzo JC, Hébert JM . A causative link between inner ear defects and long-term striatal dysfunction. Science 2013; 341: 1120–1123.
Arbogast T, Ouagazzal A-M, Chevalier C, Kopanitsa M, Afinowi N, Migliavacca E et al. Reciprocal effects on neurocognitive and metabolic phenotypes in mouse models of 16p11.2 deletion and duplication syndromes. PLoS Genet 2016; 12: e1005709.
Shiflett MW, Balleine BW . Contributions of ERK signaling in the striatum to instrumental learning and performance. Behav Brain Res 2011; 218: 240–247.
Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson T . Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology 2006; 31: 2660–2668.
Kravitz AV, Tye LD, Kreitzer AC . Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci 2012; 15: 816–818.
Shan Q, Ge M, Christie MJ, Balleine BW . The acquisition of goal-directed actions generates opposing plasticity in direct and indirect pathways in dorsomedial striatum. J Neurosci 2014; 34: 9196–9201.
Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M . Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 2014; 17: 1022–1030.
Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 2014; 494: 238–242.
Nakanishi S, Hikida T, Yawata S . Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neuroscience 2014; 282C: 49–59.
Syndrome D, Portmann T, Yang M, Mao R, Panagiotakos G, Ellegood J et al. Article behavioral abnormalities and circuit defects in the basal ganglia of a mouse model. Cell Rep 2014 7: 1077–1092.
Blizinsky KD, Diaz-Castro B, Forrest MP, Schürmann B, Bach AP, Martin-de-Saavedra MD et al. Reversal of dendritic phenotypes in 16p11.2 microduplication mouse model neurons by pharmacological targeting of a network hub. Proc Natl Acad Sci 2016; 113: 8520–8520.
Faccidomo S, Besheer J, Stanford PC, Hodge CW . Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology (Berl) 2009; 204: 135–147.
Guegan T, Cutando L, Gangarossa G, Santini E, Fisone G, Martinez A et al. Operant behavior to obtain palatable food modifies ERK activity in the brain reward circuit. Eur Neuropsychopharmacol 2013; 23: 240–252.
Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 2008; 28: 5671–5685.
Valjent E, Corbillé A-G, Bertran-Gonzalez J, Hervé D, Girault J-A . Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA 2006; 103: 2932–2937.
Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J . Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci 2000; 20: 8701–8709.
Sherr EH, Faridar A, Fregeau B, Bukshpun P, Pojman N, Thieu T et al. Abnormal ERK signaling in 16p11.2 copy number variation. Soc Neurosci Abstr 2013.
Edwards S, Bachtell RK, Guzman D, Whisler KN, Self DW . Emergence of context-associated GluR(1) and ERK phosphorylation in the nucleus accumbens core during withdrawal from cocaine self-administration. Addict Biol 2011; 16: 450–457.
Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P et al. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev 2012; 64: 65–87.
Chagniel L, Bergeron Y, Bureau G, Massicotte G, Cyr M . Regulation of tyrosine phosphatase STEP61 by protein kinase a during motor skill learning in mice. PLoS ONE 2014; 9: 1–8.
Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ . The dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci 2000; 20: 5630–5638.
Xu J, Hartley BJ, Kurup P, Phillips A, Topol A, Xu M et al. Inhibition of STEP61 ameliorates deficits in mouse and hiPSC-based schizophrenia models. Mol Psychiatry 2016; E-pub ahead of print 18 October 2016, doi: 10.1038/mp.2016.163.
Pulido R, Zúñiga A, Ullrich A . PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J 1998; 17: 7337–7350.
Lee KH, Song GJ, Kang IS, Kim SW, Paick J-S, Chung CH et al. Ubiquitin-specific protease activity of USP9Y, a male infertility gene on the Y chromosome. Reprod Fertil Dev 2003; 15: 129–133.
Bangasser DA, Curtis A, Reyes BAS, Bethea TT, Parastatidis I, Ischiropoulos H et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 2010; 15: 877, 896–904.
Kohls G, Chevallier C, Troiani V, Schultz RT . Social ‘wanting’ dysfunction in autism: neurobiological underpinnings and treatment implications. J Neurodev Disord 2012; 4: 10.
Yin HH, Ostlund SB, Knowlton BJ, Balleine BW . The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 2005; 22: 513–523.
Salamone JD, Correa M . The mysterious motivational functions of mesolimbic dopamine. Neuron 2012; 76: 470–485.
Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK et al. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell 2014; 158: 198–212.
Fuccillo MV, Földy C, Gökce Ö, Rothwell PE, Sun GL, Malenka RC et al. Single-cell mRNA profiling reveals cell-type-specific expression of neurexin isoforms. Neuron 2015; 87: 326–340.
Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011; 472: 437–442.
Zhou YY, Kaiser T, Monteiro PP, Zhang X, Van der Goes MS, Wang D et al. Mice with Shank3 mutations associated with ASD and schizophrenia display both shared and distinct defects. Neuron 2016; 89: 147–162.
Wang X, Bey AL, Katz BM, Badea A, Kim N, David LK et al. Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat Commun 2016; 7: 11459.
Wang X, Snape M, Klann E, Stone JG, Singh A, Petersen RB et al. Activation of the extracellular signal-regulated kinase pathway contributes to the behavioral deficit of fragile x-syndrome. J Neurochem 2012; 121: 672–679.
Chévere-Torres I, Kaphzan H, Bhattacharya A, Kang A, Maki JM, Gambello MJ et al. Metabotropic glutamate receptor-dependent long-term depression is impaired due to elevated ERK signaling in the δRG mouse model of tuberous sclerosis complex. Neurobiol Dis 2012; 45: 1101–1110.
Krueger DD, Osterweil EK, Chen SP, Tye LD, Bear MF . Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc Natl Acad Sci USA 2011; 108: 2587–2592.
Richter JD, Bassell GJ, Klann E . Dysregulation and restoration of translational homeostasis in fragile X syndrome. Nat Rev Neurosci 2015; 16: 595–605.
Hsu YT, Liao G, Bi X, Oka T, Tamura S, Baudry M . The PDE10A inhibitor, papaverine, differentially activates ERK in male and female rat striatal slices. Neuropharmacology 2011; 61: 1275–1281v.
Zivadinovic D, Watson CS . Membrane estrogen receptor-alpha levels predict estrogen-induced ERK1/2 activation in MCF-7 cells. Breast Cancer Res 2005; 7: R130–R144.
Bangasser Da, Valentino RJ . Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol 2012; 32: 709–723.
Carroll JC, Rosario ER, Kreimer S, Villamagna A, Gentzschein E, Stanczyk FZ et al. Sex differences in ??-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure. Brain Res 2010; 1366: 233–245v.
Cosgrove KP, Mazure CM, Staley JK . Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 2007; 62: 847–855v.
Acknowledgements
This work was funded by the Simons Foundation Autism Research Initiative (SFARI 248429, SFARI 345034 and the SFARI Undergraduate Summer Research Program). We thank the technicians Matthew Marini, Michael Heenan, Kyle White and Robert George, undergraduates Jordan Lidsky-Everson, Ariel Miller, Kevin Hershey, Nitsan Goldstein, Adewola Osunsade, Maria Navarro and Landis Walsh and the University of Pennsylvania Neurobehavioral Testing Core for helpful assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Grissom, N., McKee, S., Schoch, H. et al. Male-specific deficits in natural reward learning in a mouse model of neurodevelopmental disorders. Mol Psychiatry 23, 544–555 (2018). https://doi.org/10.1038/mp.2017.184
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.184
This article is cited by
-
Dissecting 16p11.2 hemi-deletion to study sex-specific striatal phenotypes of neurodevelopmental disorders
Molecular Psychiatry (2024)
-
Dissecting the autism-associated 16p11.2 locus identifies multiple drivers in neuroanatomical phenotypes and unveils a male-specific role for the major vault protein
Genome Biology (2023)
-
Chemogenetic rectification of the inhibitory tone onto hippocampal neurons reverts autistic-like traits and normalizes local expression of estrogen receptors in the Ambra1+/- mouse model of female autism
Translational Psychiatry (2023)
-
SARM1 deletion in parvalbumin neurons is associated with autism-like behaviors in mice
Cell Death & Disease (2022)
-
Emerging behavioral and neuroimaging biomarkers for early and accurate characterization of autism spectrum disorders: a systematic review
Translational Psychiatry (2021)