Abstract
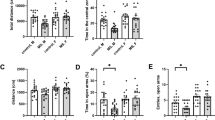
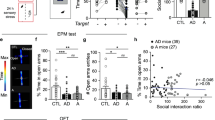
Anxiety disorders constitute a major disease and social burden worldwide; however, many questions concerning the underlying molecular mechanisms still remain open. Besides the involvement of the major excitatory (glutamate) and inhibitory (gamma aminobutyric acid (GABA)) neurotransmitter circuits in anxiety disorders, the stress system has been directly implicated in the pathophysiology of these complex mental illnesses. The glucocorticoid receptor (GR) is the major receptor for the stress hormone cortisol (corticosterone in rodents) and is widely expressed in excitatory and inhibitory neurons, as well as in glial cells. However, currently it is unknown which of these cell populations mediate GR actions that eventually regulate fear- and anxiety-related behaviors. In order to address this question, we generated mice lacking the receptor specifically in forebrain glutamatergic or GABAergic neurons by breeding GRflox/flox mice to Nex-Cre or Dlx5/6-Cre mice, respectively. GR deletion specifically in glutamatergic, but not in GABAergic, neurons induced hypothalamic–pituitary–adrenal axis hyperactivity and reduced fear- and anxiety-related behavior. This was paralleled by reduced GR-dependent electrophysiological responses in the basolateral amygdala (BLA). Importantly, viral-mediated GR deletion additionally showed that fear expression, but not anxiety, is regulated by GRs in glutamatergic neurons of the BLA. This suggests that pathological anxiety likely results from altered GR signaling in glutamatergic circuits of several forebrain regions, while modulation of fear-related behavior can largely be ascribed to GR signaling in glutamatergic neurons of the BLA. Collectively, our results reveal a major contribution of GRs in the brain’s key excitatory, but not inhibitory, neurotransmitter system in the regulation of fear and anxiety behaviors, which is crucial to our understanding of the molecular mechanisms underlying anxiety disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Norrholm SD, Ressler KJ . Genetics of anxiety and trauma-related disorders. Neuroscience 2009; 164: 272–287.
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE . Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 593–602.
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196.
Kalueff AV, Nutt DJ . Role of GABA in anxiety and depression. Depress Anxiety 2007; 24: 495–517.
Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev 2013; 37: 109–122.
Wu LJ, Kim SS, Zhuo M . Molecular targets of anxiety: from membrane to nucleus. Neurochem Res 2008; 33: 1925–1932.
Sanacora G, Treccani G, Popoli M . Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012; 62: 63–77.
Gross C, Hen R . The developmental origins of anxiety. Nat Rev Neurosci 2004; 5: 545–552.
Caspi A, Moffitt TE . Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci 2006; 7: 583–590.
Shin LM, Liberzon I . The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35: 169–191.
de Kloet ER, Joëls M, Holsboer F . Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005; 6: 463–475.
Joëls M, Baram TZ . The neuro-symphony of stress. Nat Rev Neurosci 2009; 10: 459–466.
Joëls M . Impact of glucocorticoids on brain function: relevance for mood disorders. Psychoneuroendocrinology 2011; 36: 406–414.
Yehuda R . Status of glucocorticoid alterations in post-traumatic stress disorder. Ann NY Acad Sci 2009; 1179: 56–69.
Müller MB, Holsboer F . Mice with mutations in the HPA-system as models for symptoms of depression. Biol Psychiatry 2006; 59: 1104–1115.
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 1999; 23: 99–103.
Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science 2013; 339: 332–335.
Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA 2004; 101: 11851–11856.
Wagner KV, Wang XD, Liebl C, Scharf SH, Müller MB, Schmidt MV . Pituitary glucocorticoid receptor deletion reduces vulnerability to chronic stress. Psychoneuroendocrinology 2011; 36: 579–587.
Arnett MG, Kolber BJ, Boyle MP, Muglia LJ . Behavioral insights from mouse models of forebrain—and amygdala-specific glucocorticoid receptor genetic disruption. Mol Cell Endocrinol 2011; 336: 2–5.
Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ . Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci 2006; 26: 1971–1978.
Sandi C . Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends Neurosci 2011; 34: 165–176.
Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M et al. Conditional CRH overexpressing mice: an animal model for stress-elicited pathologies and treatments that target the central CRH system. Mol Psychiatry 2008; 13: 989.
Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 1999; 24: 401–414.
Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D et al. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology 2007; 32: 417–429.
Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 2011; 333: 1903–1907.
Wang XD, Chen Y, Wolf M, Wagner KV, Liebl C, Scharf SH et al. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol Dis 2011; 42: 300–310.
Fluttert M, Dalm S, Oitzl MS . A refined method for sequential blood sampling by tail incision in rats. Lab Anim 2000; 34: 372–378.
Herman JP, Mueller NK, Figueiredo H . Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann NY Acad Sci 2004; 1018: 35–45.
Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 2001; 31: 247–260.
Day HE, Curran EJ, Watson SJ, Akil H . Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol 1999; 413: 113–128.
Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA . Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis 2006; 44: 611–621.
Klug JR, Mathur BN, Kash TL, Wang HD, Matthews RT, Robison AJ et al. Genetic inhibition of CaMKII in dorsal striatal medium spiny neurons reduces functional excitatory synapses and enhances intrinsic excitability. PLoS One 2012; 7: e45323.
Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C et al. Fluoxetine epigenetically alters the CaMKIIa promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology 2014; 39: 1178–1186.
Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci 2013; 33: 4295–4307.
Erondu NE, Kennedy MB . Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci 1985; 5: 3270–3277.
Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL et al. Distinct extended amygdala circuits for divergent motivational states. Nature 2013; 496: 224–228.
Varoqui H, Schäfer MKH, Zhu H, Weihe E, Erickson JD . Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci 2002; 22: 142–155.
Hisano S . Vesicular glutamate transporters in the brain. Anat Sci Int 2003; 78: 191–204.
Hrabovszky E, Wittmann G, Turi GF, Liposits Z, Fekete C . Hypophysiotropic thyrotropin-releasing hormone and corticotropin-releasing hormone neurons of the rat contain vesicular glutamate transporter-2. Endocrinology 2005; 146: 341–347.
Singh-Taylor A, Korosi A, Molet J, Gunn BG, Baram TZ . Synaptic rewiring of stress-sensitive neurons by early-life experience: a mechanism for resilience? Neurobiol Stress 2015; 1: 109–115.
Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 2006; 51: 455–466.
Dine J, Kühne C, Deussing JM, Eder M . Optogenetic evocation of field inhibitory postsynaptic potentials in hippocampal slices: a simple and reliable approach for studying pharmacological effects on GABAA and GABAB receptor-mediated neurotransmission. Front Cell Neurosci 2014; 8: 2.
Graham BM, Milad MR . The study of fear extinction: implications for anxiety disorders. Am J Psychiatry 2011; 168: 1255–1265.
Dias BG, Ressler KJ . Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 2014; 17: 89–96.
Bhagat SM, Butler SS, Taylor JR, McEwen BS, Strittmatter SM . Erasure of fear memories is prevented by Nogo Receptor 1 in adulthood. Mol Psychiatry; doi: 10.1038/mp.2015.179 (e-pub ahead of print 1 December 2015).
Brinks V, De Kloet ER, Oitzl MS . Corticosterone facilitates extinction of fear memory in BALB/c mice but strengthens cue related fear in C57BL/6 mice. Exp Neurol 2009; 216: 375–382.
Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci USA 2009; 106: 4888–4893.
Karst H, Berger S, Erdmann G, Schütz G, Joels M . Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA 2010; 107: 14449–14454.
McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP (ed). The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss: New York, USA, 1992, pp 69–76.
Spampanato J, Polepalli J, Sah P . Interneurons in the basolateral amygdala. Neuropharmacology 2011; 60: 765–773.
Sah P, Faber ESL, Lopez De Armentia M, Power J . The amygdaloid complex: anatomy and physiology. Physiol Rev 2003; 83: 803–834.
Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. PNAS 2005; 102: 473–478.
Laryea G, Schütz G, Muglia LJ . Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Mol Endocrinol 2013; 27: 1655–1665.
Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG . Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 2013; 7: 156.
Joels M, Karst H, DeRijk R, de Kloet ER . The coming out of the brain mineralocorticoid receptor. Trends Neurosci 2008; 31: 1–7.
Ulrich-Lai YM, Herman JP . Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 2009; 10: 397–409.
Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ . Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA 2008; 105: 12004–12009.
Blundell J, Blaiss CA, Lagace DC, Eisch AJ, Powell CM . Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiol Learn Mem 2011; 95: 453–460.
Yang YL, Chao PK, Lu KT . Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology 2006; 31: 912–924.
Myers KM, Davis M . Mechanisms of fear extinction. Mol Psychiatry 2007; 12: 120–150.
McGaugh JL, Roozendaal B . Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 2002; 12: 205–210.
Donley MP, Schulkin J, Rosen JB . Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behav Brain Res 2005; 164: 197–205.
Conrad CD, MacMillan DD, Tsekhanov S, Wright RL, Baran SE, Fuchs RA . Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiol Learn Mem 2004; 81: 185–199.
Tronel S, Alberini CM . Persistent disruption of a traumatic memory by postretrieval inactivation of glucocorticoid receptors in the amygdala. Biol Psychiatry 2007; 62: 33–39.
Acknowledgements
This work was supported by the Max Planck Society. We thank Daniela Harbich, Bianca Schmid and Ania Mederer for their excellent technical assistance; Carine Dournes for sharing unpublished data, Günther Schütz (German Cancer Research Center, Heidelberg, Germany) for originally sharing GRflox/flox mice; and Klaus-Armin Nave (Max Planck Institute of Experimental Medicine, Göttingen, Germany) for originally sharing Nex-Cre mice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Hartmann, J., Dedic, N., Pöhlmann, M. et al. Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol Psychiatry 22, 466–475 (2017). https://doi.org/10.1038/mp.2016.87
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.87
This article is cited by
-
Restoring Wnt signaling in a hormone-simulated postpartum depression model remediated imbalanced neurotransmission and depressive-like behaviors
Molecular Medicine (2023)
-
The cortisol switch between vulnerability and resilience
Molecular Psychiatry (2023)
-
Pathogenesis of Post-Traumatic Stress Disorder and Therapeutic Targets
Neuroscience and Behavioral Physiology (2023)
-
Activation of the anterior cingulate cortex ameliorates anxiety in a preclinical model of fetal alcohol spectrum disorders
Translational Psychiatry (2022)
-
Blood levels of T-Cell Receptor Excision Circles (TRECs) provide an index of exposure to traumatic stress in mice and humans
Translational Psychiatry (2022)