Abstract
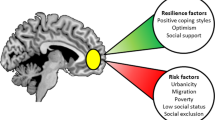
Identifying biological mechanisms through which the experience of adversity emerges as individual risk for mental illness is an important step toward developing strategies for personalized treatment and, ultimately, prevention. Preclinical studies have identified epigenetic modification of gene expression as one such mechanism. Recent clinical studies have suggested that epigenetic modification, particularly methylation of gene regulatory regions, also acts to shape human brain function associated with risk for mental illness. However, it is not yet clear whether differential gene methylation as a function of adversity contributes to the emergence of individual risk for mental illness. Using prospective longitudinal epigenetic, neuroimaging and behavioral data from 132 adolescents, we demonstrate that changes in gene methylation associated with lower socioeconomic status (SES) predict changes in risk-related brain function. Specifically, we find that lower SES during adolescence is associated with an increase in methylation of the proximal promoter of the serotonin transporter gene, which predicts greater increases in threat-related amygdala reactivity. We subsequently demonstrate that greater increases in amygdala reactivity moderate the association between a positive family history for depression and the later manifestation of depressive symptoms. These initial results suggest a specific biological mechanism through which adversity contributes to altered brain function, which in turn moderates the emergence of general liability as individual risk for mental illness. If replicated, this prospective pathway may represent a novel target biomarker for intervention and prevention among high-risk individuals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF . Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 2004; 82: 217–225.
Goodman E, Slap GB, Huang B . The public health impact of socioeconomic status on adolescent depresssion and obesity. Am J Public Health 2003; 93: 1844–1850.
Cohen S, Janicki-Deverts D, Chen E, Matthews KA . Childhood socioeconomic status and adult health. Ann NY Acad Sci 2010; 1186: 37–55.
Fryers T, Melzer D, Jenkins R . Social inequalities and the common mental disorders: a systematic review of the evidence. Soc Psychiatry Psychiatr Epidemiol 2003; 38: 229–237.
Barbeau EM, Krieger N, Soobader M . Working class matters: Socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health 2010; 94: 269–278.
Bagot RC, Meaney MJ . Epigenetics and the biological basis of gene x environment interactions. J Am Acad Child Adolesc Psychiatry 2010; 49: 752–771.
McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 2009; 12: 342–348.
Beach SR, Brody GH, Lei MK, Kim S, Cui J, Philibert RA . Is serotonin transpoter genotype associated with epigenetic susceptibility or vulnerability? Examination of the impact of socioeconomic status risk on African American youth. Dev Psychopathol 2014; 26: 289–304.
Nikolova YS, Hariri AR . Can we observe epigenetic effects on human brain function? Trends Cogn Sci 2015; 19: 366–373.
Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA . Methylation at SLC6A4 is linked to family history of child abuse: An examination of the Iowa Adoptee sample. Am J Med Genet B Neuropsychiatr Genet 2010; 153B: 710–713.
Beach SRH, Dogan MV, Brody GH, Philibert RA . Differential impact of cumulative SES risk on methylation of protein-protein interaction pathways as a function of SLC6A4 genetic variation in African American young adults. Biol Psychol 2014; 96: 28–34.
Zhao J, Goldberg J, Bremner JD, Vaccarino V . Association between promoter methylation of serotonin transpoter gene and depressive symptoms: a monozygotic twin study. Psychosom Med 2013; 75: 523–529.
Nikolova YS, Koenen KC, Galea S, Wang C, Seney ML, Sibille E et al. Beyond genotype: Serotonin transporter epigenetic modification predicts human brain function. Nat Neurosci 2014; 17: 1153–1155.
Swartz JR, Knodt AR, Radtke SR, Hariri AR . A neural biomarker of psychological vulnerability to future life stress. Neuron 2015; 85: 505–511.
Hariri AR, Holmes A . Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends Cogn Sci 2006; 10: 182–191.
Swartz JR, Williamson DE, Hariri AR . Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. Am J Psychiatry 2015; 172: 276–283.
Williamson DE, Birmaher B, Axelson D, Ryan ND, Dahl RE . First episode of depression in children at low and high familial risk for depression. J Am Acad Child Adolesc Psychiatry 2004; 43: 291–297.
Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H . Offspring of depressed parents: 20 years later. Am J Psychiatry 2006; 163: 1001–1008.
Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H et al. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proc Natl Acad Sci USA 2009; 106: 14120–14125.
McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M et al. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress Anxiety 2014; 31: 834–842.
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D . Principal component analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38: 904–909.
Hanson JL, Hariri AR, Williamson DE . Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol Psychiatry 2015; 78: 598–605.
Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 2003; 27: 169–190.
Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci 2007; 2: 161–173.
Williamson DE, Birmaher B, Ryan ND, Shiffrin TP, Lusky JA, Protopapa J et al. The stressful life events schedule for children and adolescents: development and validation. Psychiatry Res 2003; 119: 225–241.
Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005; 210: 343–352.
Achenbach TM . Manual for the Youth Self-Report and 1991 Profiles. Department of Psychiatry, University of Vermont: Burlington, VT, 1991.
Achenbach TM, Dumenci L, Rescorla LA . DSM-oriented and empirically based approaches to constructing scales from the same item pools. J Clin Child Adolesc Psychol 2003; 32: 328–340.
Ferdinand RF . Validity of the CBCL/YSR DSM-IV scales anxiety problems and affective problems. J Anxiety Disord 2008; 22: 126–134.
Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S et al. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolsecent offspring. Am J Med Genet B Neuropsychiatr Genet 2010; 153: 1350–1354.
Burdge GC, Lillycrop KA, Phillips ES, Slater-Jefferies JL, Jackson AA, Hanson MA . Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr 2009; 139: 1054–1060.
Lutz J, Herwig U, Opialla S, HIttmeyer A, Jancke L, Rufer M et al. Mindfulness and emotion regulation—an fMRI study. Soc Cogn Affect Neurosci 2013; 9: 776–785.
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism grant R01AA016274 and the Dielmann Family (DEW), MH087493 (Consuelo Walss-Bass, PhD), the National Institute on Drug Abuse grant R01DA033369 and R01DA031579 (ARH), the National Institute on Aging grant R01AG049789 (ARH) and the Center for the Study of Adolescent Risk and Resilience, NIH grant P30DA023026 (JRS). We thank Ryan Bogdan, Annchen Knodt and Caitlin Carey for assistance with conducting analyses and Yuliya Nikolova for discussions of the manuscript.
Author contributions
ARH and DEW designed the study; DEW oversaw analysis of DNA methylation; ARH and JRS oversaw analysis of functional imaging data; JRS conducted the statistical analyses and drafted the manuscript; all authors edited and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Swartz, J., Hariri, A. & Williamson, D. An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents. Mol Psychiatry 22, 209–214 (2017). https://doi.org/10.1038/mp.2016.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.82
This article is cited by
-
An epigenome-wide analysis of socioeconomic position and tumor DNA methylation in breast cancer patients
Clinical Epigenetics (2023)
-
A systematic review of neuroimaging epigenetic research: calling for an increased focus on development
Molecular Psychiatry (2023)
-
Biomarkers in Child and Adolescent Depression
Child Psychiatry & Human Development (2023)
-
An avoidable crisis
Harm Reduction Journal (2022)
-
Shedding light on biological sex differences and microbiota–gut–brain axis: a comprehensive review of its roles in neuropsychiatric disorders
Biology of Sex Differences (2022)