Abstract
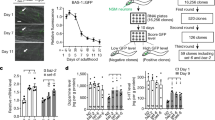
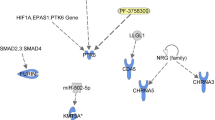
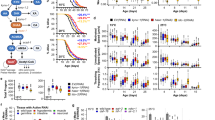
Antidepressants have been shown to improve longevity in C. elegans. It is plausible that orthologs of genes involved in mood regulation and stress response are involved in such an effect. We sought to understand the underlying biology. First, we analyzed the transcriptome from worms treated with the antidepressant mianserin, previously identified in a large-scale unbiased drug screen as promoting increased lifespan in worms. We identified the most robust treatment-related changes in gene expression, and identified the corresponding human orthologs. Our analysis uncovered a series of genes and biological pathways that may be at the interface between antidepressant effects and longevity, notably pathways involved in drug metabolism/degradation (nicotine and melatonin). Second, we examined which of these genes overlap with genes which may be involved in depressive symptoms in an aging non-psychiatric human population (n=3577), discovered using a genome-wide association study (GWAS) approach in a design with extremes of distribution of phenotype. Third, we used a convergent functional genomics (CFG) approach to prioritize these genes for relevance to mood disorders and stress. The top gene identified was ANK3. To validate our findings, we conducted genetic and gene-expression studies, in C. elegans and in humans. We studied C. elegans inactivating mutants for ANK3/unc-44, and show that they survive longer than wild-type, particularly in older worms, independently of mianserin treatment. We also show that some ANK3/unc-44 expression is necessary for the effects of mianserin on prolonging lifespan and survival in the face of oxidative stress, particularly in younger worms. Wild-type ANK3/unc-44 increases in expression with age in C. elegans, and is maintained at lower youthful levels by mianserin treatment. These lower levels may be optimal in terms of longevity, offering a favorable balance between sufficient oxidative stress resistance in younger worms and survival effects in older worms. Thus, ANK3/unc-44 may represent an example of antagonistic pleiotropy, in which low-expression level in young animals are beneficial, but the age-associated increase becomes detrimental. Inactivating mutations in ANK3/unc-44 reverse this effect and cause detrimental effects in young animals (sensitivity to oxidative stress) and beneficial effect in old animals (increased survival). In humans, we studied if the most significant single nucleotide polymorphism (SNP) for depressive symptoms in ANK3 from our GWAS has a relationship to lifespan, and show a trend towards longer lifespan in individuals with the risk allele for depressive symptoms in men (odds ratio (OR) 1.41, P=0.031) but not in women (OR 1.08, P=0.33). We also examined whether ANK3, by itself or in a panel with other top CFG-prioritized genes, acts as a blood gene-expression biomarker for biological age, in two independent cohorts, one of live psychiatric patients (n=737), and one of suicide completers from the coroner’s office (n=45). We show significantly lower levels of ANK3 expression in chronologically younger individuals than in middle age individuals, with a diminution of that effect in suicide completers, who presumably have been exposed to more severe and acute negative mood and stress. Of note, ANK3 was previously reported to be overexpressed in fibroblasts from patients with Hutchinson–Gilford progeria syndrome, a form of accelerated aging. Taken together, these studies uncover ANK3 and other genes in our dataset as biological links between mood, stress and longevity/aging, that may be biomarkers as well as targets for preventive or therapeutic interventions. Drug repurposing bioinformatics analyses identified the relatively innocuous omega-3 fatty acid DHA (docosahexaenoic acid), piracetam, quercetin, vitamin D and resveratrol as potential longevity promoting compounds, along with a series of existing drugs, such as estrogen-like compounds, antidiabetics and sirolimus/rapamycin. Intriguingly, some of our top candidate genes for mood and stress-modulated longevity were changed in expression in opposite direction in previous studies in the Alzheimer disease. Additionally, a whole series of others were changed in expression in opposite direction in our previous studies on suicide, suggesting the possibility of a “life switch” actively controlled by mood and stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chesney E, Goodwin GM, Fazel S . Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 2014; 13: 153–160.
Lohr JB, Palmer BW, Eidt CA, Aailaboyina S, Mausbach BT, Wolkowitz OM et al. Is post-traumatic stress disorder associated with premature senescence? A review of the literature. Am J Geriatr Psychiatry 2015; 23: 709–725.
Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ . Does growing old increase the risk for depression? Am J Psychiatry 1997; 154: 1384–1390.
Coppen A, Kopera H . Workshop on the clinical pharmacology and efficacy of mianserin. Br J Clin Pharmacol 1978; 5: 91 S–99 S.
Moreau JL, Bourson A, Jenck F, Martin JR, Mortas P . Curative effects of the atypical antidepressant mianserin in the chronic mild stress-induced anhedonia model of depression. J Psychiatry Neurosci 1994; 19: 51–56.
Petrascheck M, Ye X, Buck LB . An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 2007; 450: 553–556.
Rangaraju S, Solis GM, Thompson RC, Gomez-Amaro RL, Kurian L, Encalada SE et al. Suppression of transcriptional drift extends C. elegans lifespan by postponing the onset of mortality. eLife 2015; 4: e08833.
Nho K, Ramanan VK, Horgusluoglu E, Kim S, Inlow MH, Risacher SL et al. Comprehensive gene- and pathway-based analysis of depressive symptoms in older adults. J Alzheimers Dis 2015; 45: 1197–1206.
Levey DF, Le-Niculescu H, Frank J, Ayalew M, Jain N, Kirlin B et al. Genetic risk prediction and neurobiological understanding of alcoholism. Transl Psychiatry 2014; 4: e391.
Ogden CA, Rich ME, Schork NJ, Paulus MP, Geyer MA, Lohr JB et al. Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol Psychiatry 2004; 9: 1007–1029.
Le-Niculescu H, McFarland MJ, Ogden CA, Balaraman Y, Patel S, Tan J et al. Phenomic, convergent functional genomic, and biomarker studies in a stress-reactive genetic animal model of bipolar disorder and co-morbid alcoholism. Am J Med Genet B Neuropsychiatr Genet 2008; 147B: 134–166.
Le-Niculescu H, Case NJ, Hulvershorn L, Patel SD, Bowker D, Gupta J et al. Convergent functional genomic studies of omega-3 fatty acids in stress reactivity, bipolar disorder and alcoholism. Transl Pychiatry 2011; 1: e4.
Niculescu AB, Le-Niculescu H . Convergent functional genomics: what we have learned and can learn about genes, pathways, and mechanisms. Neuropsychopharmacology 2010; 35: 355–356.
Zhang EE, Liu AC, Hirota T, Miraglia LJ, Welch G, Pongsawakul PY et al. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell 2009; 139: 199–210.
McCarthy MJ, Welsh DK . Cellular circadian clocks in mood disorders. J Biol Rhythms 2012; 27: 339–352.
Niculescu AB, Levey DF, Phalen PL, Le-Niculescu H, Dainton HD, Jain N et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry 2015; 20: 1266–1285.
Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 2006; 313: 1929–1935.
Musiek ES . Circadian clock disruption in neurodegenerative diseases: cause and effect? Front Pharmacol 2015; 6: 29.
Wolkove N, Elkholy O, Baltzan M, Palayew M . Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ 2007; 176: 1299–1304.
Maes OC, Xu S, Yu B, Chertkow HM, Wang E, Schipper HM . Transcriptional profiling of Alzheimer blood mononuclear cells by microarray. Neurobiol Aging 2007; 28: 1795–1809.
Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ . Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res 2002; 70: 462–473.
Nead KT, Gaskin G, Chester C, Swisher-McClure S, Dudley JT, Leeper NJ et al. Androgen deprivation therapy and future alzheimer's disease risk. J Clin Oncol 2016; 34: 566–571.
Kim S, Choi KH, Baykiz AF, Gershenfeld HK . Suicide candidate genes associated with bipolar disorder and schizophrenia: an exploratory gene expression profiling analysis of post-mortem prefrontal cortex. BMC Genomics 2007; 8: 413.
Le-Niculescu H, Levey DF, Ayalew M, Palmer L, Gavrin LM, Jain N et al. Discovery and validation of blood biomarkers for suicidality. Mol Psychiatry 2013; 18: 1249–1264.
Carretero M, Gomez-Amaro RL, Petrascheck M . Pharmacological classes that extend lifespan of Caenorhabditis elegans. Front Genet 2015; 6: 77.
Smith KR, Kopeikina KJ, Fawcett-Patel JM, Leaderbrand K, Gao R, Schurmann B et al. Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron 2014; 84: 399–415.
Tseng WC, Jenkins PM, Tanaka M, Mooney R, Bennett V . Giant ankyrin-G stabilizes somatodendritic GABAergic synapses through opposing endocytosis of GABAA receptors. Proc Natl Acad Sci USA 2015; 112: 1214–1219.
Rueckert EH, Barker D, Ruderfer D, Bergen SE, O'Dushlaine C, Luce CJ et al. Cis-acting regulation of brain-specific ANK3 gene expression by a genetic variant associated with bipolar disorder. Mol Psychiatry 2013; 18: 922–929.
Chen H, Wang N, Zhao X, Ross CA, O'Shea KS, McInnis MG . Gene expression alterations in bipolar disorder postmortem brains. Bipolar Disord 2013; 15: 177–187.
Wirgenes KV, Tesli M, Inderhaug E, Athanasiu L, Agartz I, Melle I et al. ANK3 gene expression in bipolar disorder and schizophrenia. Br J Psychiatry 2014; 205: 244–245.
Kato T, Hayashi-Takagi A, Toyota T, Yoshikawa T, Iwamoto K . Gene expression analysis in lymphoblastoid cells as a potential biomarker of bipolar disorder. J Hum Genet 2011; 56: 779–783.
Leussis MP, Berry-Scott EM, Saito M, Jhuang H, de Haan G, Alkan O et al. The ANK3 bipolar disorder gene regulates psychiatric-related behaviors that are modulated by lithium and stress. Biol Psychiatry 2013; 73: 683–690.
Ignatiuk A, Quickfall JP, Hawrysh AD, Chamberlain MD, Anderson DH . The smaller isoforms of ankyrin 3 bind to the p85 subunit of phosphatidylinositol 3'-kinase and enhance platelet-derived growth factor receptor down-regulation. J Biol Chem 2006; 281: 5956–5964.
Durak O, de Anda FC, Singh KK, Leussis MP, Petryshen TL, Sklar P et al. Ankyrin-G regulates neurogenesis and Wnt signaling by altering the subcellular localization of beta-catenin. Mol Psychiatry 2015; 20: 388–397.
Edinger RS, Coronnello C, Bodnar AJ, LaFramboise WA, Benos PV, Ho J et al. Aldosterone regulates microRNAs in the cortical collecting duct to alter sodium transport. J Am Soc Nephrol 2014; 25: 2445–2457.
Kapahi P . Protein synthesis and the antagonistic pleiotropy hypothesis of aging. Adv Exp Med Biol 2010; 694: 30–37.
Blackburn EH, Epel ES, Lin J . Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 2015; 350: 1193–1198.
Johnson SC, Rabinovitch PS, Kaeberlein M . mTOR is a key modulator of ageing and age-related disease. Nature 2013; 493: 338–345.
Bratic A, Larsson NG . The role of mitochondria in aging. J Clin Investig 2013; 123: 951–957.
Chen DT, Jiang X, Akula N, Shugart YY, Wendland JR, Steele CJ et al. Genome-wide association study meta-analysis of European and Asian-ancestry samples identifies three novel loci associated with bipolar disorder. Mol Psychiatry 2013; 18: 195–205.
Takata A, Kim SH, Ozaki N, Iwata N, Kunugi H, Inada T et al. Association of ANK3 with bipolar disorder confirmed in East Asia. Am J Med Genet B 2011; 156B: 312–315.
Psychiatric GCBDWG. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet 2011; 43: 977–983.
Lim CH, Zain SM, Reynolds GP, Zain MA, Roffeei SN, Zainal NZ et al. Genetic association of LMAN2L gene in schizophrenia and bipolar disorder and its interaction with ANK3 gene polymorphism. Prog Neuropsychopharmacol Biol Psychiatry 2014; 54: 157–162.
Lee MT, Chen CH, Lee CS, Chen CC, Chong MY, Ouyang WC et al. Genome-wide association study of bipolar I disorder in the Han Chinese population. Mol Psychiatry 2011; 16: 548–556.
Logue MW, Solovieff N, Leussis MP, Wolf EJ, Melista E, Baldwin C et al. The ankyrin-3 gene is associated with posttraumatic stress disorder and externalizing comorbidity. Psychoneuroendocrinology 2013; 38: 2249–2257.
Le-Niculescu H, Case NJ, Hulvershorn L, Patel SD, Bowker D, Gupta J et al. Convergent functional genomic studies of omega-3 fatty acids in stress reactivity, bipolar disorder and alcoholism. Transl Psychiatry 2011; 1: e4.
Wellcome Trust Case Control C.. Genome-wide association study of 14 000 cases of seven common diseases and 3000 shared controls. Nature 2007; 447: 661–678.
Turner CA, Thompson RC, Bunney WE, Schatzberg AF, Barchas JD, Myers RM et al. Altered choroid plexus gene expression in major depressive disorder. Front Hum Neurosci 2014; 8: 238.
Beech RD, Lowthert L, Leffert JJ, Mason PN, Taylor MM, Umlauf S et al. Increased peripheral blood expression of electron transport chain genes in bipolar depression. Bipolar Disord 2010; 12: 813–824.
Gray JD, Rubin TG, Hunter RG, McEwen BS . Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry 2014; 19: 1171–1178.
Kubota M, Kasahara T, Iwamoto K, Komori A, Ishiwata M, Miyauchi T et al. Therapeutic implications of down-regulation of cyclophilin D in bipolar disorder. Int J Neuropsychopharmacol 2010; 13: 1355–1368.
Su YA, Wu J, Zhang L, Zhang Q, Su DM, He P et al. Dysregulated mitochondrial genes and networks with drug targets in postmortem brain of patients with posttraumatic stress disorder (PTSD) revealed by human mitochondria-focused cDNA microarrays. Int J Biol Sci 2008; 4: 223–235.
Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry 2008; 64: 266–272.
Nakamura K, Chen CK, Sekine Y, Iwata Y, Anitha A, Loh el W et al. Association analysis of SOD2 variants with methamphetamine psychosis in Japanese and Taiwanese populations. Hum Genet 2006; 120: 243–252.
McAuley EZ, Blair IP, Liu Z, Fullerton JM, Scimone A, Van Herten M et al. A genome screen of 35 bipolar affective disorder pedigrees provides significant evidence for a susceptibility locus on chromosome 15q25-26. Mol Psychiatry 2009; 14: 492–500.
Gaiteri C, Guilloux JP, Lewis DA, Sibille E . Altered gene synchrony suggests a combined hormone-mediated dysregulated state in major depression. PLoS One 2010; 5: e9970.
Zhurov V, Stead JD, Merali Z, Palkovits M, Faludi G, Schild-Poulter C et al. Molecular pathway reconstruction and analysis of disturbed gene expression in depressed individuals who died by suicide. PLoS One 2012; 7: e47581.
Martins-de-Souza D, Maccarrone G, Ising M, Kloiber S, Lucae S, Holsboer F et al. Blood mononuclear cell proteome suggests integrin and Ras signaling as critical pathways for antidepressant treatment response. Biol Psychiatry 2014; 76: e15–e17.
Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G et al. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology 2009; 34: 1363–1380.
Ewald H, Flint T, Kruse TA, Mors O . A genome-wide scan shows significant linkage between bipolar disorder and chromosome 12q24.3 and suggestive linkage to chromosomes 1p22-21, 4p16, 6q14-22, 10q26 and 16p13.3. Mol Psychiatry 2002; 7: 734–744.
Vawter MP, Tomita H, Meng F, Bolstad B, Li J, Evans S et al. Mitochondrial-related gene expression changes are sensitive to agonal-pH state: implications for brain disorders. Mol Psychiatry 2006; 11: 663–679.
Bhasin MK, Dusek JA, Chang BH, Joseph MG, Denninger JW, Fricchione GL et al. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS One 2013; 8: e62817.
McQuillin A, Rizig M, Gurling HM . A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet Genomics 2007; 17: 605–617.
Velders FP, Kuningas M, Kumari M, Dekker MJ, Uitterlinden AG, Kirschbaum C et al. Genetics of cortisol secretion and depressive symptoms: a candidate gene and genome wide association approach. Psychoneuroendocrinology 2011; 36: 1053–1061.
Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G . Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc Natl Acad Sci USA 2008; 105: 5567–5572.
Zubenko GS, Maher B, Hughes HB 3rd, Zubenko WN, Stiffler JS, Kaplan BB et al. Genome-wide linkage survey for genetic loci that influence the development of depressive disorders in families with recurrent, early-onset, major depression. Am J Med Genet B Neuropsychiatr Genet 2003; 123: 1–18.
Doyle AE, Biederman J, Ferreira MA, Wong P, Smoller JW, Faraone SV . Suggestive linkage of the child behavior checklist juvenile bipolar disorder phenotype to 1p21, 6p21, and 8q21. J Am Acad Child Adolesc Psychiatry 2010; 49: 378–387.
Le-Niculescu H, Kurian SM, Yehyawi N, Dike C, Patel SD, Edenberg HJ et al. Identifying blood biomarkers for mood disorders using convergent functional genomics. Mol Psychiatry 2009; 14: 156–174.
Morita K, Saito T, Ohta M, Ohmori T, Kawai K, Teshima-Kondo S et al. Expression analysis of psychological stress-associated genes in peripheral blood leukocytes. Neurosci Lett 2005; 381: 57–62.
Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT . Social regulation of gene expression in human leukocytes. Genome Biol 2007; 8: R189.
Kang HJ, Adams DH, Simen A, Simen BB, Rajkowska G, Stockmeier CA et al. Gene expression profiling in postmortem prefrontal cortex of major depressive disorder. J Neurosci 2007; 27: 13329–13340.
Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem 2008; 105: 921–932.
Acknowledgements
This work is, in essence, a field-wide collaboration. We acknowledge our debt of gratitude for the efforts and results of the many other groups, cited in our paper, who have conducted and published studies (clinical, genetic and biological) in mood disorders, stress, longevity and aging. With their arduous and careful work, a convergent approach such as ours is possible. We thank Farnoosh Khan, Naga Vanipenta and Eddie Stage for help with building literature databases. We also would particularly like to thank the subjects who participated in these studies, their families and their caregivers. Without their contribution, such work to advance the understanding of aging would not be possible. This work was supported by two NIH Directors’ New Innovator Awards (1DP2OD007363 to ABN and 1DP2OD008398 to MP), as well as NIH U19 A1063603 to DRS, NIH R00 LM011384 to KN and IADC P30 AG010133 to AJS.
Author contributions
ABN, MP, DRS and AJS designed the study, and ABN wrote the manuscript. SR, DFL, KN, NJ, KDA and HLN conducted experiments and analyzed the data. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest. ABN, MP, DRS and AJS are listed as inventors on a patent application being filed by Indiana University and Scripps.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website .
Supplementary information
Rights and permissions
About this article
Cite this article
Rangaraju, S., Levey, D., Nho, K. et al. Mood, stress and longevity: convergence on ANK3. Mol Psychiatry 21, 1037–1049 (2016). https://doi.org/10.1038/mp.2016.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.65
This article is cited by
-
Lithium rescues dendritic abnormalities in Ank3 deficiency models through the synergic effects of GSK3β and cyclic AMP signaling pathways
Neuropsychopharmacology (2023)
-
Kif15 deficiency contributes to depression-like behavior in mice
Metabolic Brain Disease (2023)
-
Precision medicine in psychiatry: biomarkers to the forefront
Neuropsychopharmacology (2022)
-
Precision medicine for mood disorders: objective assessment, risk prediction, pharmacogenomics, and repurposed drugs
Molecular Psychiatry (2021)
-
Blood biomarkers for memory: toward early detection of risk for Alzheimer disease, pharmacogenomics, and repurposed drugs
Molecular Psychiatry (2020)