Abstract
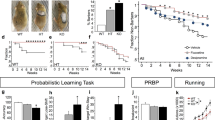
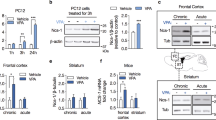
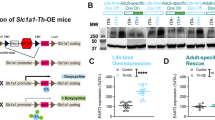
Manic episodes are one of the major diagnostic symptoms in a spectrum of neuropsychiatric disorders that include schizophrenia, obsessive-compulsive disorder and bipolar disorder (BD). Despite a possible association between BD and the gene encoding phospholipase Cγ1 (PLCG1), its etiological basis remains unclear. Here, we report that mice lacking phospholipase Cγ1 (PLCγ1) in the forebrain (Plcg1f/f; CaMKII) exhibit hyperactivity, decreased anxiety-like behavior, reduced depressive-related behavior, hyperhedonia, hyperphagia, impaired learning and memory and exaggerated startle responses. Inhibitory transmission in hippocampal pyramidal neurons and striatal dopamine receptor D1-expressing neurons of Plcg1-deficient mice was significantly reduced. The decrease in inhibitory transmission is likely due to a reduced number of γ-aminobutyric acid (GABA)-ergic boutons, which may result from impaired localization and/or stabilization of postsynaptic CaMKII (Ca2+/calmodulin-dependent protein kinase II) at inhibitory synapses. Moreover, mutant mice display impaired brain-derived neurotrophic factor-tropomyosin receptor kinase B-dependent synaptic plasticity in the hippocampus, which could account for deficits of spatial memory. Lithium and valproate, the drugs presently used to treat mania associated with BD, rescued the hyperactive phenotypes of Plcg1f/f; CaMKII mice. These findings provide evidence that PLCγ1 is critical for synaptic function and plasticity and that the loss of PLCγ1 from the forebrain results in manic-like behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Greer PL, Greenberg ME . From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron 2008; 59: 846–860.
Rantamaki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, Domenici E et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cγ signaling pathways in mouse brain. Neuropsychopharmacology 2007; 32: 2152–2162.
Gu B, Huang YZ, He XP, Joshi RB, Jang W, McNamara JO . A peptide uncoupling BDNF receptor TrkB from phospholipase Cγ1 prevents epilepsy induced by status epilepticus. Neuron 2015; 88: 484–491.
Turecki G, Grof P, Cavazzoni P, Duffy A, Grof E, Ahrens B et al. Evidence for a role of phospholipase C-γ1 in the pathogenesis of bipolar disorder. Mol Psychiatry 1998; 3: 534–538.
Lovlie R, Berle JO, Stordal E, Steen VM . The phospholipase C-γ1 gene (PLCG1) and lithium-responsive bipolar disorder: re-examination of an intronic dinucleotide repeat polymorphism. Psychiatr Genet 2001; 11: 41–43.
Serretti A, Mandelli L . The genetics of bipolar disorder: genome 'hot regions,' genes, new potential candidates and future directions. Mol Psychiatry 2008; 13: 742–771.
Nurnberger JI Jr, Koller DL, Jung J, Edenberg HJ, Foroud T, Guella I et al. Identification of pathways for bipolar disorder: a meta-analysis. JAMA Psychiatry 2014; 71: 657–664.
Hou L, Heilbronner U, Degenhardt F, Adli M, Akiyama K, Akula N et al. Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study. Lancet 2016; 387: 1085–1093.
Radhakrishna U, Senol S, Herken H, Gucuyener K, Gehrig C, Blouin JL et al. An apparently dominant bipolar affective disorder (BPAD) locus on chromosome 20p11.2-q11.2 in a large Turkish pedigree. Eur J Hum Genet 2001; 9: 39–44.
Berridge MJ, Downes CP, Hanley MR . Neural and developmental actions of lithium: a unifying hypothesis. Cell 1989; 59: 411–419.
Park H, Poo MM . Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 2013; 14: 7–23.
Okada T, Hashimoto R, Numakawa T, Iijima Y, Kosuga A, Tatsumi M et al. A complex polymorphic region in the brain-derived neurotrophic factor (BDNF) gene confers susceptibility to bipolar disorder and affects transcriptional activity. Mol Psychiatry 2006; 11: 695–703.
Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim Y, Tsan G et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry 2002; 7: 579–593.
Chen G, Henter ID, Manji HK . Translational research in bipolar disorder: emerging insights from genetically based models. Mol Psychiatry 2010; 15: 883–895.
Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 1999; 24: 401–414.
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 1999; 23: 99–103.
Jia Y, Zhou J, Tai Y, Wang Y . TRPC channels promote cerebellar granule neuron survival. Nat Neurosci 2007; 10: 559–567.
Trovo L, Ahmed T, Callaerts-Vegh Z, Buzzi A, Bagni C, Chuah M et al. Low hippocampal PI(4,5)P-2 contributes to reduced cognition in old mice as a result of loss of MARCKS. Nat Neurosci 2013; 16: 449–455.
Ji QS, Winnier GE, Niswender KD, Horstman D, Wisdom R, Magnuson MA et al. Essential role of the tyrosine kinase substrate phospholipase C-γ1 in mammalian growth and development. Proc Natl Acad Sci USA 1997; 94: 2999–3003.
Gruart A, Sciarretta C, Valenzuela-Harrington M, Delgado-Garcia JM, Minichiello L . Mutation at the TrkB PLCγ-docking site affects hippocampal LTP and associative learning in conscious mice. Learn Mem 2007; 14: 54–62.
Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M . Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 2002; 36: 121–137.
Bender RE, Alloy LB . Life stress and kindling in bipolar disorder: review of the evidence and integration with emerging biopsychosocial theories. Clin Psychol Rev 2011; 31: 383–398.
Leussis MP, Berry-Scott EM, Saito M, Jhuang H, de Haan G, Alkan O et al. The ANK3 bipolar disorder gene regulates psychiatric-related behaviors that are modulated by lithium and stress. Biol Psychiatry 2013; 73: 683–690.
Le-Niculescu H, McFarland MJ, Ogden CA, Balaraman Y, Patel S, Tan J et al. Phenomic, convergent functional genomic, and biomarker studies in a stress-reactive genetic animal model of bipolar disorder and co-morbid alcoholism. Am J Med Genet B 2008; 147B: 134–166.
Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010; 466: 622–626.
Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF . Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry 2004; 9: 609–620.
Hu H, Gan J, Jonas P . Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science 2014; 345: 1255263.
Korte M, Minichiello L, Klein R, Bonhoeffer T . SHC-binding site in the TRKB receptor is not required tor hippocampal long-term potentiation. Neuropharmacology 2000; 39: 717–724.
Gartner A, Polnau DG, Staiger V, Sciarretta C, Minichiello L, Thoenen H et al. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cγ signaling. J Neurosci 2006; 26: 3496–3504.
Kang H, Welcher AA, Shelton D, Schuman EM . Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron 1997; 19: 653–664.
Patterson SL, Pittenger C, Morozov A, Martin KC, Scanlin H, Drake C et al. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron 2001; 32: 123–140.
Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng L et al. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci 2010; 13: 302–309.
Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA 2010; 107: 2687–2692.
Rose CR, Blum R, Pichler B, Lepier A, Kafitz KW, Konnerth A . Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature 2003; 426: 74–78.
Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 1994; 12: 11–24.
van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL et al. Phospholipase Cγ1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature 2005; 434: 99–104.
Houston CM, He Q, Smart TG . CaMKII phosphorylation of the GABA(A) receptor: receptor subtype- and synapse-specific modulation. J Physiol 2009; 587: 2115–2125.
Klausberger T, Roberts JDB, Somogyi P . Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci 2002; 22: 2513–2521.
Brickley SG, Mody I . Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 2012; 73: 23–34.
Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G . Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999; 397: 259–263.
Scheffer RE . Concurrent ADHD and bipolar disorder. Curr Psychiatry Rep 2007; 9: 415–419.
Barkley RA . Attention Deficit Hyperactivity Disorder in Adults: The Latest Assessment and Treatment Strategies, vol. vi. Jones and Bartlett Publishers: Sudbury, MA, USA, 2010, p 81.
Won H, Mah W, Kim E, Kim JW, Hahm EK, Kim MH et al. GIT1 is associated with ADHD in humans and ADHD-like behaviors in mice. Nat Med 2011; 17: 566–572.
Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS et al. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry 2000; 157: 1108–1114.
Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol psychiatry 2008; 13: 858–872.
Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry 2009; 14: 448–461.
Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA 2007; 104: 6406–6411.
Han K, Holder JL Jr, Schaaf CP, Lu H, Chen H, Kang H et al. SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature 2013; 503: 72–77.
Ryan MM, Lockstone HE, Huffaker SJ, Wayland MT, Webster MJ, Bahn S . Gene expression analysis of bipolar disorder reveals downregulation of the ubiquitin cycle and alterations in synaptic genes. Mol Psychiatry 2006; 11: 965–978.
Ting JT, Peca J, Feng G . Functional consequences of mutations in postsynaptic scaffolding proteins and relevance to psychiatric disorders. Annu Rev Neurosci 2012; 35: 49–71.
Minichiello L . TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 2009; 10: 850–860.
Frerking M, Malenka RC, Nicoll RA . Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol 1998; 80: 3383–3386.
Marsden KC, Shemesh A, Bayer KU, Carroll RC . Selective translocation of Ca2+/calmodulin protein kinase IIalpha (CaMKIIalpha) to inhibitory synapses. Proc Natl Acad Sci USA 2010; 107: 20559–20564.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011; 477: 171–178.
Uhlhaas PJ, Singer W . Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron 2012; 75: 963–980.
Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M . Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA 2007; 104: 10164–10169.
Yong W, Zhang MM, Wang S, Ruan DY . Effects of sodium valproate on synaptic transmission and neuronal excitability in rat hippocampus. Clin Exp Pharmacol Physiol 2009; 36: 1062–1067.
Lee SH, Sohn JW, Ahn SC, Park WS, Ho WK . Li+ enhances GABAergic inputs to granule cells in the rat hippocampal dentate gyrus. Neuropharmacology 2004; 46: 638–646.
Mei L, Nave KA . Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 2014; 83: 27–49.
Thomson PA, Christoforou A, Morris SW, Adie E, Pickard BS, Porteous DJ et al. Association of neuregulin 1 with schizophrenia and bipolar disorder in a second cohort from the Scottish population. Mol Psychiatry 2007; 12: 94–104.
Meier S, Strohmaier J, Breuer R, Mattheisen M, Degenhardt F, Muhleisen TW et al. Neuregulin 3 is associated with attention deficits in schizophrenia and bipolar disorder. Int J Neuropsychopharmacol 2013; 16: 549–556.
Yang YR, Choi JH, Chang JS, Kwon HM, Jang HJ, Ryu SH et al. Diverse cellular and physiological roles of phospholipase C-γ1. Adv Biol Regul 2012; 52: 138–151.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) Grant, funded by the Korean Government (MOE) (2013R1A1A2064434) and a grant by the Korean Government (MSIP) (2010-0028684 and 2007-341-C00027; to P-GS), and by NRF Grants (2014051826, 2015R1A2A1A15054037 and 2015M3C7A1027351; to J-HK). We thank MP Kong at POSTECH for supporting generation of PLCγ1 conditional knockout mice, YH Lee at UNIST for maintaining mice and technical support, JH Hur at UNIST-Olympus Biomedical imaging Center (UOBC) for technical support and M Suh at the Korea Institute of Science and Technology (KIST) for experimental support for the behavior test. We also thank CH Bailey (Neuroscience, Columbia University) for critical reading and comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Yang, Y., Jung, J., Kim, SJ. et al. Forebrain-specific ablation of phospholipase Cγ1 causes manic-like behavior. Mol Psychiatry 22, 1473–1482 (2017). https://doi.org/10.1038/mp.2016.261
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.261
This article is cited by
-
PLCγ1 in dopamine neurons critically regulates striatal dopamine release via VMAT2 and synapsin III
Experimental & Molecular Medicine (2023)
-
The druggable schizophrenia genome: from repurposing opportunities to unexplored drug targets
npj Genomic Medicine (2022)
-
MXenes nanocomposites for energy storage and conversion
Rare Metals (2022)
-
Long non-coding RNAs in brain tumors: roles and potential as therapeutic targets
Journal of Hematology & Oncology (2021)
-
Dysfunction of ventral tegmental area GABA neurons causes mania-like behavior
Molecular Psychiatry (2021)