Abstract
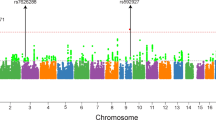
Many schizophrenia susceptibility loci have been identified through genome-wide association studies (GWASs) in European populations. However, until recently, schizophrenia GWASs in non-European populations were limited to small sample sizes and have yielded few loci associated with schizophrenia. To identify genetic risk variations for schizophrenia in the Han Chinese population, we performed a two-stage GWAS of schizophrenia comprising 4384 cases and 5770 controls, followed by independent replications of 13 single-nucleotide polymorphisms in an additional 4339 schizophrenia cases and 7043 controls of Han Chinese ancestry. Furthermore, we conducted additional analyses based on the results in the discovery stage. The combined analysis confirmed evidence of genome-wide significant associations in the Han Chinese population for three loci, at 2p16.1 (rs1051061, in an exon of VRK2, P=1.14 × 10−12, odds ratio (OR)=1.17), 6p22.1 (rs115070292 in an intron of GABBR1, P=4.96 × 10−10, OR=0.77) and 10q24.32 (rs10883795 in an intron of AS3MT, P=7.94 × 10−10, OR=0.87; rs10883765 at an intron of ARL3, P=3.06 × 10−9, OR=0.87). The polygenic risk score based on Psychiatric Genomics Consortium schizophrenia GWAS data modestly predicted case–control status in the Chinese population (Nagelkerke R2: 1.7% ~5.7%). Our pathway analysis suggested that neurological biological pathways such as GABAergic signaling, dopaminergic signaling, cell adhesion molecules and myelination pathways are involved in schizophrenia. These findings provide new insights into the pathogenesis of schizophrenia in the Han Chinese population. Further studies are needed to establish the biological context and potential clinical utility of these findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Thaker GK, Carpenter WT Jr . Advances in schizophrenia. Nat Med 2001; 7: 667–671.
Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 2009; 373: 234–239.
International Schizophrenia C, Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460: 748–752.
Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 2009; 460: 753–757.
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D et al. Common variants conferring risk of schizophrenia. Nature 2009; 460: 744–747.
Shi Y, Li Z, Xu Q, Wang T, Li T, Shen J et al. Common variants on 8p12 and 1q24.2 confer risk of schizophrenia. Nat Genet 2011; 43: 1224–1227.
Yue WH, Wang HF, Sun LD, Tang FL, Liu ZH, Zhang HX et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2. Nat Genet 2011; 43: 1228–1231.
Ripke S, O'Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet 2013; 45: 1150–1159.
Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427.
Wong EH, So HC, Li M, Wang Q, Butler AW, Paul B et al. Common variants on Xq28 conferring risk of schizophrenia in Han Chinese. Schizophr Bull 2014; 40: 777–786.
Howie BN, Donnelly P, Marchini J . A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009; 5: e1000529.
Delaneau O, Zagury JF, Marchini J . Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods 2013; 10: 5–6.
Marchini J, Howie B . Genotype imputation for genome-wide association studies. Nat Rev Genet 2010; 11: 499–511.
de Bakker PI, Ferreira MA, Jia X, Neale BM, Raychaudhuri S, Voight BF . Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum Mol Genet 2008; 17: R122–R128.
Skol AD, Scott LJ, Abecasis GR, Boehnke M . Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet 2006; 38: 209–213.
Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 2010; 26: 2336–2337.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Ramasamy A, Trabzuni D, Guelfi S, Varghese V, Smith C, Walker R et al. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci 2014; 17: 1418–1428.
Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M et al. Spatio-temporal transcriptome of the human brain. Nature 2011; 478: 483–489.
Yang J, Lee SH, Goddard ME, Visscher PM . GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 2011; 88: 76–82.
Li MX, Gui HS, Kwan JS, Sham PC . GATES: a rapid and powerful gene-based association test using extended Simes procedure. Am J Hum Genet 2011; 88: 283–293.
Li MX, Kwan JS, Sham PC . HYST: a hybrid set-based test for genome-wide association studies, with application to protein-protein interaction-based association analysis. Am J Hum Genet 2012; 91: 478–488.
Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF . GABA(B(1)) receptor isoforms differentially mediate the acquisition and extinction of aversive taste memories. J Neurosci 2006; 26: 8800–8803.
Jacobson LH, Bettler B, Kaupmann K, Cryan JF . Behavioral evaluation of mice deficient in GABA(B(1)) receptor isoforms in tests of unconditioned anxiety. Psychopharmacology 2007; 190: 541–553.
Li M, Jaffe AE, Straub RE, Tao R, Shin JH, Wang Y et al. A human-specific AS3MT isoform and BORCS7 are molecular risk factors in the 10q24.32 schizophrenia-associated locus. Nat Med 2016; 22: 649–656.
Sanchez-Pena LC, Petrosyan P, Morales M, Gonzalez NB, Gutierrez-Ospina G, Del Razo LM et al. Arsenic species, AS3MT amount, and AS3MT gene expression in different brain regions of mouse exposed to arsenite. Environ Res 2010; 110: 428–434.
Aberg KA, Liu Y, Bukszar J, McClay JL, Khachane AN, Andreassen OA et al. A comprehensive family-based replication study of schizophrenia genes. JAMA Psychiatry 2013; 70: 573–581.
Steinberg S, de Jong S, Irish Schizophrenia Genomics C Andreassen OA, Werge T, Borglum AD et al. Common variants at VRK2 and TCF4 conferring risk of schizophrenia. Hum Mol Genet 2011; 20: 4076–4081.
Li M, Wang Y, Zheng XB, Ikeda M, Iwata N, Luo XJ et al. Meta-analysis and brain imaging data support the involvement of VRK2 (rs2312147) in schizophrenia susceptibility. Schizophr Res 2012; 142: 200–205.
Blanco S, Sanz-Garcia M, Santos CR, Lazo PA . Modulation of interleukin-1 transcriptional response by the interaction between VRK2 and the JIP1 scaffold protein. PloS One 2008; 3: e1660.
Fernandez IF, Perez-Rivas LG, Blanco S, Castillo-Dominguez AA, Lozano J, Lazo PA . VRK2 anchors KSR1-MEK1 to endoplasmic reticulum forming a macromolecular complex that compartmentalizes MAPK signaling. Cell Mol Life Sci 2012; 69: 3881–3893.
Dajas-Bailador F, Jones EV, Whitmarsh AJ . The JIP1 scaffold protein regulates axonal development in cortical neurons. Curr Biol 2008; 18: 221–226.
Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature 2012; 489: 57–74.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P et al. A method and server for predicting damaging missense mutations. Nat Methods 2010; 7: 248–249.
Deutsch SI, Burket JA, Katz E . Does subtle disturbance of neuronal migration contribute to schizophrenia and other neurodevelopmental disorders? Potential genetic mechanisms with possible treatment implications. Eur Neuropsychopharmacol 2010; 20: 281–287.
Ishizuka K, Kamiya A, Oh EC, Kanki H, Seshadri S, Robinson JF et al. DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature 2011; 473: 92–96.
Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA et al. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron 2010; 67: 33–48.
Valiente M, Marin O . Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol 2010; 20: 68–78.
Barnat M, Enslen H, Propst F, Davis RJ, Soares S, Nothias F . Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J Neurosci 2010; 30: 7804–7816.
White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 1998; 396: 679–682.
Chen CM, Stanford AD, Mao X, Abi-Dargham A, Shungu DC, Lisanby SH et al. GABA level, gamma oscillation, and working memory performance in schizophrenia. NeuroImage Clin 2014; 4: 531–539.
Fatemi SH, Folsom TD, Thuras PD . Deficits in GABA(B) receptor system in schizophrenia and mood disorders: a postmortem study. Schizophr Res 2011; 128: 37–43.
Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J et al. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell 2012; 148: 1051–1064.
Wassef A, Baker J, Kochan LD . GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol 2003; 23: 601–640.
Cavenagh MM, Breiner M, Schurmann A, Rosenwald AG, Terui T, Zhang C et al. ADP-ribosylation factor (ARF)-like 3, a new member of the ARF family of GTP-binding proteins cloned from human and rat tissues. J Biol Chem 1994; 269: 18937–18942.
Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z et al. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med 2007; 232: 3–13.
Agusa T, Fujihara J, Takeshita H, Iwata H . Individual variations in inorganic arsenic metabolism associated with AS3MT genetic polymorphisms. Int J Mol Sci 2011; 12: 2351–2382.
Reichard JF, Puga A . Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics 2010; 2: 87–104.
Lou HM . An investigation of the heritability of schizophrenia in Northeast China. Chin J Neurol Psychiatry 1983; 16: 49–50.
Kwon E, Wang W, Tsai LH . Validation of schizophrenia-associated genes CSMD1, C10orf26, CACNA1C and TCF4 as miR-137 targets. Mol Psychiatry 2013; 18: 11–12.
Havik B, Le Hellard S, Rietschel M, Lybaek H, Djurovic S, Mattheisen M et al. The complement control-related genes CSMD1 and CSMD2 associate to schizophrenia. Biol Psychiatry 2011; 70: 35–42.
Chen J, Wang M, Waheed Khan RA, He K, Wang Q, Li Z et al. The GSK3B gene confers risk for both major depressive disorder and schizophrenia in the Han Chinese population. J Affect Disord 2015; 185: 149–155.
Acknowledgements
We express our sincere gratitude to all subjects who participated in the study. We acknowledge the schizophrenia working group of the Psychiatric GWAS Consortium (PGC) for making their results publicly available. This work was supported by grants from the National Key Technology R&D Program of China (2015BAI13B01), the National Natural Science Foundation of China (91232305, 81361120395, 91432304, 81571313) and the National High Technology Research and Development Program of China (2009AA022702).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Yu, H., Yan, H., Li, J. et al. Common variants on 2p16.1, 6p22.1 and 10q24.32 are associated with schizophrenia in Han Chinese population. Mol Psychiatry 22, 954–960 (2017). https://doi.org/10.1038/mp.2016.212
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.212
This article is cited by
-
Sex effects on DNA methylation affect discovery in epigenome-wide association study of schizophrenia
Molecular Psychiatry (2024)
-
Reduced Vrk2 expression is associated with higher risk of depression in humans and mediates depressive-like behaviors in mice
BMC Medicine (2023)
-
Mendelian Randomization Study Using Dopaminergic Neuron-Specific eQTL Identifies Novel Risk Genes for Schizophrenia
Molecular Neurobiology (2023)
-
Proteome-wide Mendelian randomization reveals the causal effects of immune-related plasma proteins on psychiatric disorders
Human Genetics (2023)
-
Involvement of the long intergenic non-coding RNA LINC00461 in schizophrenia
BMC Psychiatry (2022)