Abstract
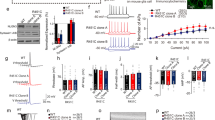
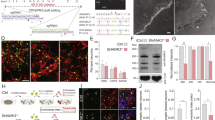
Neuroligins are postsynaptic cell-adhesion molecules that bind to presynaptic neurexins. Mutations in neuroligin-3 predispose to autism, but how such mutations affect synaptic function remains incompletely understood. Here we systematically examined the effect of three autism-associated mutations, the neuroligin-3 knockout, the R451C knockin, and the R704C knockin, on synaptic transmission in the calyx of Held, a central synapse ideally suited for high-resolution analyses of synaptic transmission. Surprisingly, germline knockout of neuroligin-3 did not alter synaptic transmission, whereas the neuroligin-3 R451C and R704C knockins decreased and increased, respectively, synaptic transmission. These puzzling results prompted us to ask whether neuroligin-3 mutant phenotypes may be reshaped by developmental plasticity. Indeed, conditional knockout of neuroligin-3 during late development produced a marked synaptic phenotype, whereas conditional knockout of neuroligin-3 during early development caused no detectable effect, mimicking the germline knockout. In canvassing potentially redundant candidate genes, we identified developmentally early expression of another synaptic neurexin ligand, cerebellin-1. Strikingly, developmentally early conditional knockout of cerebellin-1 only modestly impaired synaptic transmission, whereas in contrast to the individual single knockouts, developmentally early conditional double knockout of both cerebellin-1 and neuroligin-3 severely decreased synaptic transmission. Our data suggest an unanticipated mechanism of developmental compensation whereby cerebellin-1 and neuroligin-3 functionally occlude each other during development of calyx synapses. Thus, although acute manipulations more likely reveal basic gene functions, developmental plasticity can be a major factor in shaping the overall phenotypes of genetic neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Südhof TC . Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008; 455: 903–911.
Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM . LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci 2010; 30: 7495–7506.
Yuzaki M . Cbln1 and its family proteins in synapse formation and maintenance. Curr Opin Neurobiol 2011; 21: 215–220.
Song JY, Ichtchenko K, Südhof TC, Brose N . Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA 1999; 96: 1100–1105.
Varoqueaux F, Jamain S, Brose N . Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol 2004; 83: 449–456.
Budreck EC, Scheiffele P . Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci 2007; 26: 1738–1748.
Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt K-F et al. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc Natl Acad Sci USA 2011; 108: 3053–3058.
Takács VT, Freund TF, Nyiri G . Neuroligin 2 is expressed in synapses established by cholinergic cells in the mouse brain. PLoS ONE 2013; 8: e72450.
Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK et al. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell 2014; 158: 198–212.
Liang J, Xu W, Hsu Y-T, Yee A, Chen L, Südhof T . Conditional neuroligin-2 knockout in adult medial prefrontal cortex links chronic changes in synaptic inhibition to cognitive impairments. Mol Psychiatry 2015; 20: 850–859.
Zhang B, Chen LY, Liu X, Maxeiner S, Lee S-J, Gokce O et al. Neuroligins sculpt cerebellar purkinje-cell circuits by differential control of distinct classes of synapses. Neuron 2015; 87: 781–796.
Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 2003; 34: 27–29.
Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 williams syndrome region, are strongly associated with autism. Neuron 2011; 70: 863–885.
Levy D, Ronemus M, Yamrom B, Lee Yha, Leotta A, Kendall J et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 2011; 70: 886–897.
Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 2007; 318: 71–76.
Etherton M, Földy C, Sharma M, Tabuchi K, Liu X, Shamloo M et al. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci USA 2011; 108: 13764–13769.
Földy C, Malenka RC, Südhof TC . Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron 2013; 78: 498–509.
Hirai H, Pang Z, Bao D, Miyazaki T, Li L, Miura E et al. Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat Neurosci 2005; 8: 1534–1541.
Mizuno Y, Takahashi K, Totsune K, Ohneda M, Konno H, Murakami O et al. Decrease in cerebellin and corticotropin-releasing hormone in the cerebellum of olivopontocerebellar atrophy and Shy-Drager syndrome. Brain Res 1995; 686: 115–118.
Boghosian-Sell L, Comings DE, Overhauser J . Tourette syndrome in a pedigree with a 7;18 translocation: identification of a YAC spanning the translocation breakpoint at 18q22.3. Am J Hum Genet 1996; 59: 999–1005.
Abalkhail H, Mitchell J, Habgood J, Orrell R, de Belleroche J . A new familial amyotrophic lateral sclerosis locus on chromosome 16q12.1-16q12.2. Am J Hum Genet 2003; 73: 383–389.
Clarke Ra, Lee S, Eapen V . Pathogenetic model for Tourette syndrome delineates overlap with related neurodevelopmental disorders including Autism. Transl Psychiatry 2012; 2: e163.
Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M et al. Trans-synaptic interaction of GluRδ2 and neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 2010; 141: 1068–1079.
Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S et al. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science (80-) 2010; 328: 363–368.
Kusnoor SV, Parris J, Muly EC, Morgan JI, Deutch a Y . Extracerebellar role for Cerebellin1: modulation of dendritic spine density and synapses in striatal medium spiny neurons. J Comp Neurol 2010; 518: 2525–2537.
Miura E, Iijima T, Yuzaki M, Watanabe M . Distinct expression of Cbln family mRNAs in developing and adult mouse brains. Eur J Neurosci 2006; 24: 750–760.
Wei P, Smeyne RJ, Bao D, Parris J, Morgan JI . Mapping of Cbln1-like immunoreactivity in adult and developing mouse brain and its localization to the endolysosomal compartment of neurons. Eur J Neurosci 2007; 26: 2962–2978.
Yu WM, Goodrich LV . Morphological and physiological development of auditory synapses. Hear Res 2014; 311: 3–16.
Borst JGG, Soria van Hoeve J . The calyx of held synapse: from model synapse to auditory relay. Annu Rev Physiol 2012; 74: 199–224.
Nakamura PA, Cramer KS . Formation and maturation of the calyx of Held. Hear Res 2011; 276: 70–78.
Wang L-Y, Fedchyshyn MJ, Yang Y-M . Action potential evoked transmitter release in central synapses: insights from the developing calyx of Held. Mol Brain 2009; 2: 36.
Takahashi T . Strength and precision of neurotransmission at mammalian presynaptic terminals. Proc Jpn Acad Ser B Phys Biol Sci 2015; 91: 305–320.
von Gersdorff H, Borst JGG . Short-term plasticity at the calyx of held. Nat Rev Neurosci 2002; 3: 53–64.
Holcomb PS, Hoffpauir BK, Hoyson MC, Jackson DR, Deerinck TJ, Marrs GS et al. Synaptic inputs compete during rapid formation of the calyx of held: a new model system for neural development. J Neurosci 2013; 33: 12954–12969.
Xiao L, Michalski N, Kronander E, Gjoni E, Genoud C, Knott G et al. BMP signaling specifies the development of a large and fast CNS synapse. Nat Neurosci 2013; 16: 856–864.
Abdul-Latif ML, Salazar JAA, Marshak S, Dinh ML, Cramer KS . Ephrin-A2 and ephrin-A5 guide contralateral targeting but not topographic mapping of ventral cochlear nucleus axons. Neural Dev 2015; 10: 27.
Yang Y-M, Fedchyshyn MJ, Grande G, Aitoubah J, Tsang CW, Xie H et al. Septins regulate developmental switching from microdomain to nanodomain coupling of Ca(2+) influx to neurotransmitter release at a central synapse. Neuron 2010; 67: 100–115.
Michalski N, Babai N, Renier N, Perkel DJ, Ch??dotal A, Schneggenburger R . Robo3-driven axon midline crossing conditions functional maturation of a large commissural synapse. Neuron 2013; 78: 855–868.
Aoto J, Martinelli DC, Malenka RC, Tabuchi K, Südhof TC . Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell 2013; 154: 75–88.
Zhang B, Sun L, Yang Y-M, Huang H-P, Zhu F-P, Wang L et al. Action potential bursts enhance transmitter release at a giant central synapse. J Physiol 2011; 589: 2213–2227.
Acuna C, Liu X, Gonzalez A, Südhof TC . RIM-BPs mediate tight coupling of action potentials to Ca2+-triggered neurotransmitter release. Neuron 2015; 87: 1234–1247.
Forsythe ID, Barnes-Davies M . The binaural auditory pathway: membrane currents limiting multiple action potential generation in the rat medial nucleus of the trapezoid body. Proc Biol Sci 1993; 251: 143–150.
Schneggenburger R, Meyer AC, Neher E . Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron 1999; 23: 399–409.
Etherton MR, Tabuchi K, Sharma M, Ko J, Südhof TC . An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs AMPA receptor-mediated synaptic transmission in hippocampus. EMBO J 2011; 30: 2908–2919.
Chanda S, Aoto J, Lee S-J, Wernig M, Südhof TC . Pathogenic mechanism of an autism-associated neuroligin mutation involves altered AMPA-receptor trafficking. Mol Psychiatry 2015; 21: 169–177.
Voiculescu O, Charnay P, Schneider-Maunoury S . Expression pattern of a Krox-20/Cre knock-in allele in the developing hindbrain, bones, and peripheral nervous system. Genesis 2000; 26: 123–126.
De S, Shuler CF, Turman JE . The ontogeny of Krox-20 expression in brainstem and cerebellar neurons. J Chem Neuroanat 2003; 25: 213–226.
Han Y, Kaeser PS, Südhof TC, Schneggenburger R . RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron 2011; 69: 304–316.
Yang Y-M, Aitoubah J, Lauer AM, Nuriya M, Takamiya K, Jia Z et al. GluA4 is indispensable for driving fast neurotransmission across a high-fidelity central synapse. J Physiol 2011; 589: 4209–4227.
Huguet G, Ey E, Bourgeron T . The genetic landscapes of autism spectrum disorders. Annu Rev Genomics Hum Genet 2013; 14: 191–213.
Ichtchenko K, Nguyen T, Südhof TC . Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem 1996; 271: 2676–2682.
Chih B, Gollan L, Scheiffele P . Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron 2006; 51: 171–178.
Comoletti D, Flynn RE, Boucard AA, Demeler B, Schirf V, Shi J et al. Gene selection, alternative splicing, and post-translational processing regulate neuroligin selectivity for β-neurexins. Biochemistry 2006; 45: 12816–12827.
Lilley BN, Krishnaswamy A, Wang Z, Kishi M, Frank E, Sanes JR . SAD kinases control the maturation of nerve terminals in the mammalian peripheral and central nervous systems. Proc Natl Acad Sci USA 2014; 111: 1138–1143.
Kolson DR, Wan J, Wu J, Dehoff M, Brandebura AN, Qian J et al. Temporal patterns of gene expression during calyx of held development. Dev Neurobiol 2016; 76: 166–189.
Wallace DC . Mitochondrial diseases in man and mouse. Science 1999; 283: 1482–1488.
Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 10: 8192–8192.
McCarthy SE, Gillis J, Kramer M, Lihm J, Yoon S, Berstein Y et al. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol Psychiatry 2014; 19: 652–658.
Guilmatre A, Huguet G, Delorme R, Bourgeron T . The emerging role of SHANK genes in neuropsychiatric disorders. Dev Neurobiol 2014; 74: 113–122.
Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 2013; 45: 984–994.
Group C, Consortium PG. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 2013; 381: 1371–1379.
Kenny EM, Cormican P, Furlong S, Heron E, Kenny G, Fahey C et al. Excess of rare novel loss-of-function variants in synaptic genes in schizophrenia and autism spectrum disorders. Mol Psychiatry 2014; 19: 872–879.
Smoller JW, Finn CT . Family, twin, and adoption studies of bipolar disorder. Am J Med Genet Part C Semin Med Genet 2003; 123C: 48–58.
Maier W, Lichtermann D, Minges J, Hallmayer J, Heun R, Benkert O et al. Continuity and discontinuity of affective disorders and schizophrenia: Results of a controlled family study. Arch Gen Psychiatry 1993; 50: 871–883.
Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 2009; 373: 234–239.
Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N . Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry 2009; 48: 10–18.
King BH, Lord C . Is schizophrenia on the autism spectrum? Brain Res 2011; 1380: 34–41.
Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J et al. Copy number variants in schizophrenia: Confirmation of five previous finding sand new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry 2011; 168: 302–316.
Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R . Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J Child Psychol Psychiatry Allied Discip 2008; 49: 535–542.
Rommelse NNJ, Franke B, Geurts HM, Hartman CA, Buitelaar JK . Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry 2010; 19: 281–295.
Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H . The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry 2010; 167: 1357–1363.
Williams NM, Franke B, Mick E, Anney RJL, Freitag CM, Gill M et al. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: The role of rare variants and duplications at 15q13.3. Am J Psychiatry 2012; 169: 195–204.
Acknowledgements
We thank Drs Sungjin Lee and Salome Botelho for the help of Cbln1 purification and RT-PCR, and members of the Südhof labs for valuable discussions. This study was supported by a grant from the Simons Foundation Autism Research Initiative (307762, to TCS) and from NIMH (R37MH052804, to TCS). Correspondence and requests for materials should be addressed to BZ (zbo@stanford.edu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, B., Seigneur, E., Wei, P. et al. Developmental plasticity shapes synaptic phenotypes of autism-associated neuroligin-3 mutations in the calyx of Held. Mol Psychiatry 22, 1483–1491 (2017). https://doi.org/10.1038/mp.2016.157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.157
This article is cited by
-
Analyses of the autism-associated neuroligin-3 R451C mutation in human neurons reveal a gain-of-function synaptic mechanism
Molecular Psychiatry (2022)
-
Genetics of glutamate and its receptors in autism spectrum disorder
Molecular Psychiatry (2022)
-
Neuroligin-3 Regulates Excitatory Synaptic Transmission and EPSP-Spike Coupling in the Dentate Gyrus In Vivo
Molecular Neurobiology (2022)
-
Imbalance of flight–freeze responses and their cellular correlates in the Nlgn3−/y rat model of autism
Molecular Autism (2022)
-
Comprehensive somatosensory and neurological phenotyping of NCS1 knockout mice
Scientific Reports (2021)