Abstract
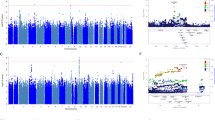
Up to 30% of patients with obsessive-compulsive disorder (OCD) exhibit an inadequate response to serotonin reuptake inhibitors (SRIs). To date, genetic predictors of OCD treatment response have not been systematically investigated using genome-wide association study (GWAS). To identify specific genetic variations potentially influencing SRI response, we conducted a GWAS study in 804 OCD patients with information on SRI response. SRI response was classified as ‘response’ (n=514) or ‘non-response’ (n=290), based on self-report. We used the more powerful Quasi-Likelihood Score Test (the MQLS test) to conduct a genome-wide association test correcting for relatedness, and then used an adjusted logistic model to evaluate the effect size of the variants in probands. The top single-nucleotide polymorphism (SNP) was rs17162912 (P=1.76 × 10−8), which is near the DISP1 gene on 1q41-q42, a microdeletion region implicated in neurological development. The other six SNPs showing suggestive evidence of association (P<10−5) were rs9303380, rs12437601, rs16988159, rs7676822, rs1911877 and rs723815. Among them, two SNPs in strong linkage disequilibrium, rs7676822 and rs1911877, located near the PCDH10 gene, gave P-values of 2.86 × 10−6 and 8.41 × 10−6, respectively. The other 35 variations with signals of potential significance (P<10−4) involve multiple genes expressed in the brain, including GRIN2B, PCDH10 and GPC6. Our enrichment analysis indicated suggestive roles of genes in the glutamatergic neurotransmission system (false discovery rate (FDR)=0.0097) and the serotonergic system (FDR=0.0213). Although the results presented may provide new insights into genetic mechanisms underlying treatment response in OCD, studies with larger sample sizes and detailed information on drug dosage and treatment duration are needed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
American Psychiatric Association (ed). Diagnostic and statistical manual of mental disorders (DSM-IV). Psychiatric Press: Washington, 1994.
Nestadt G, Grados M, Samuels JF . Genetics of obsessive-compulsive disorder. Psychiatr Clin North Am 2010; 33: 141–158.
Shugart YY, Samuels J, Willour VL, Grados MA, Greenberg BD, Knowles JA et al. Genomewide linkage scan for obsessive-compulsive disorder: evidence for susceptibility loci on chromosomes 3q, 7p, 1q, 15q, and 6q. Mol Psychiatry 2006; 11: 763–770.
Shugart YY, Wang Y, Samuels JF, Grados MA, Greenberg BD, Knowles JA et al. A family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder in 378 families. Am J Med Genet B Neuropsychiatr Genet 2009; 150B: 886–892.
Voyiaziakis E, Evgrafov O, Li D, Yoon HJ, Tabares P, Samuels J et al. Association of SLC6A4 variants with obsessive-compulsive disorder in a large multicenter US family study. Mol Psychiatry 2012; 16: 108–120.
Murphy DL, Lesch KP . Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci 2008; 9: 85–96.
Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA et al. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology (Berl) 2004; 174: 530–538.
Stewart SE FJ, Moorjani J, Jenike E, Beattie K, Illmann C, Delorme R et al Family-Based Association between Obsessive-Compulsive Disorder and Glutamate Receptor Candidate Genes. World Congress of Psychiatric Genetics: New York, 2007.
Alonso P, Gratacos M, Segalas C, Escaramis G, Real E, Bayes M et al. Association between the NMDA glutamate receptor GRIN2B gene and obsessive-compulsive disorder. J Psychiatry Neurosci 2012; 37: 273–281.
Taylor S . Molecular genetics of obsessive-compulsive disorder: a comprehensive meta-analysis of genetic association studies. Mol Psychiatry 2013; 18: 799–805.
Stewart SE, Yu D, Scharf JM, Neale BM, Fagerness JA, Mathews CA et al. Genome-wide association study of obsessive-compulsive disorder. Mol Psychiatry 2013; 18: 788–798.
Mattheisen M, Samuels JF, Wang Y, Greenberg BD, Fyer AJ, McCracken JT et al. Genome-wide association study in obsessive-compulsive disorder: results from the OCGAS. Mol Psychiatry 2014; 20: 337–344.
Ferguson JM . SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry 2001; 3: 22–27.
Di Bella D, Erzegovesi S, Cavallini MC, Bellodi L . Obsessive-compulsive disorder, 5-HTTLPR polymorphism and treatment response. Pharmacogenomics J 2002; 2: 176–181.
Brandl EJ, Tiwari AK, Zhou X, Deluce J, Kennedy JL, Muller DJ et al. Influence of CYP2D6 and CYP2C19 gene variants on antidepressant response in obsessive-compulsive disorder. Pharmacogenomics J 2014; 14: 176–181.
Brandl EJ, Muller DJ, Richter MA . Pharmacogenetics of obsessive-compulsive disorders. Pharmacogenomics 2012; 13: 71–81.
Sangkuhl K, Klein TE, Altman RB . Selective serotonin reuptake inhibitors pathway. Pharmacogenet Genomics 2009; 19: 907–909.
Tansey KE, Guipponi M, Perroud N, Bondolfi G, Domenici E, Evans D et al. Genetic predictors of response to serotonergic and noradrenergic antidepressants in major depressive disorder: a genome-wide analysis of individual-level data and a meta-analysis. PLoS Med 2012; 9: e1001326.
Samuels JF, Riddle MA, Greenberg BD, Fyer AJ, McCracken JT, Rauch SL et al. The OCD collaborative genetics study: methods and sample description. Am J Med Genet B Neuropsychiatr Genet 2006; 141B: 201–207.
Nestadt G, Wang Y, Grados MA, Riddle MA, Greenberg BD, Knowles JA et al. Homeobox genes in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet 2011; 159B: 53–60.
Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989; 46: 1006–1011.
Thornton T, McPeek MS . Case-control association testing with related individuals: a more powerful quasi-likelihood score test. Am J Hum Genet 2007; 81: 321–337.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Chelala C, Khan A, Lemoine NR . SNPnexus: a web database for functional annotation of newly discovered and public domain single nucleotide polymorphisms. Bioinformatics 2009; 25: 655–661.
Hindorff LA MJ, Morales J, Junkins HA, Hall PN, Klemm AK, Manolio TA. et al. A Catalog of Published Genome-Wide Association Studies. URL www.genome.gov/gwastudies Accessed on 23 Jan 2013.
Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 2010; 26: 2336–2337.
Purcell S, Cherny SS, Sham PC . Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 2003; 19: 149–150.
Dennis G Jr ., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 2003; 4: P3.
Segre AV, Groop L, Mootha VK, Daly MJ, Altshuler D . Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS Genet 2010; 6: e1001058.
Etheridge LA, Crawford TQ, Zhang S, Roelink H . Evidence for a role of vertebrate Disp1 in long-range Shh signaling. Development 2010; 137: 133–140.
Kim SY, Chung HS, Sun W, Kim H . Spatiotemporal expression pattern of non-clustered protocadherin family members in the developing rat brain. Neuroscience 2007; 147: 996–1021.
Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 2012; 486: 410–414.
Jun KR, Hur YJ, Lee JN, Kim HR, Shin JH, Oh SH et al. Clinical characterization of DISP1 haploinsufficiency: A case report. Eur J Med Genet 2013; 56: 309–313.
Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS et al. Identifying autism loci and genes by tracing recent shared ancestry. Science 2008; 321: 218–223.
Redies C, Hertel N, Hubner CA . Cadherins and neuropsychiatric disorders. Brain Res 2012; 1470: 130–144.
Arnold PD, Macmaster FP, Hanna GL, Richter MA, Sicard T, Burroughs E et al. Glutamate system genes associated with ventral prefrontal and thalamic volume in pediatric obsessive-compulsive disorder. Brain Imaging Behav 2009; 3: 64–76.
Cai J, Zhang W, Yi Z, Lu W, Wu Z, Chen J et al. Influence of polymorphisms in genes SLC1A1, GRIN2B, and GRIK2 on clozapine-induced obsessive-compulsive symptoms. Psychopharmacology (Berl) 2013; 230: 49–55.
Davis KL, Charney D, Coyle JT, Nemeroff C (eds) Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins: Philadelphia, 2002.
Korf BR, Rehm HL . New approaches to molecular diagnosis. JAMA 2013; 309: 1511–1521.
Acknowledgements
This project is a multiple sites collaborative project of OCD Collaborative Genetics Association Study (OCGAS), which is a collaboration among investigators at seven sites in the United States (namely Brown University, Columbia University, University of Southern California, Johns Hopkins University, Massachusetts General Hospital, University of California at Los Angeles, and the National Institute of Mental Health) funded by NIMH Grant Numbers: MH071507, MH079489, MH079487, MH079488 and MH079494. Qin and Shugart are both supported by IRP (Project number MH002930-04). The views expressed in this presentation do not necessarily represent the views of the NIMH, NIH, HHS or the United States Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Qin, H., Samuels, J., Wang, Y. et al. Whole-genome association analysis of treatment response in obsessive-compulsive disorder. Mol Psychiatry 21, 270–276 (2016). https://doi.org/10.1038/mp.2015.32
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.32
This article is cited by
-
Psychotic Vulnerability and its Associations with Clinical Characteristics in Adolescents with Obsessive-Compulsive Disorder
Research on Child and Adolescent Psychopathology (2023)
-
The role of Pcdh10 in neurological disease and cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Do polygenic risk and stressful life events predict pharmacological treatment response in obsessive compulsive disorder? A gene–environment interaction approach
Translational Psychiatry (2019)