Abstract
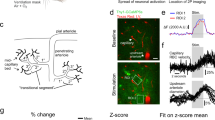
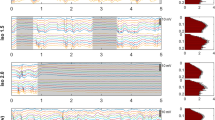
Cocaine affects neuronal activity and constricts cerebral blood vessels, making it difficult to determine whether cocaine-induced changes in cerebral blood flow (CBF) reflect neuronal activation or its vasoactive effects. Here we assessed the effects of acute cocaine on both resting-state and stimulation responses to investigate cocaine’s effects on neurovascular coupling and to differentiate its effects on neuronal activity from its vasoactive actions. We concurrently measured cortical field potentials via thinned-skull electroencephalography recordings and CBF with laser Doppler flowmetry in the rat’s somatosensory cortex for both resting state and forepaw stimulation before and following cocaine administration (1 mg kg−1, intravenously). Results show both resting-state field potentials and CBF were depressed after cocaine administration (19.8±4.7% and 52.1±13.4%, respectively) and these changes were strongly correlated with each other (r=0.81, P<0.001), indicating that cocaine did not affect neurovascular coupling at rest and that the reduction in resting CBF reflected reduction in synchronized spontaneous neuronal activity rather than vasoconstriction. In contrast, the forepaw stimulation-evoked neuronal activity was not changed by cocaine (P=0.244), whereas the CBF to the stimulation was reduced 49.9±2.6% (P=0.028) gradually recovering ∼20 min after cocaine injection, indicating that neurovascular coupling during stimulation was temporarily disrupted by cocaine. Neurovascular uncoupling by cocaine during stimulation but not during rest indicates that distinct processes might underlie neurovascular regulation for both stimulation and spontaneous activity. The greater reductions by cocaine to the stimulation-induced CBF increases than to the background CBF should be considered when interpreting functional MRI studies comparing activation responses between controls and cocaine abusers. Neurovascular uncoupling could contribute to cocaine’s neurotoxicity, particularly for stimulation conditions when CBF might be insufficient to cover for the energetic demands of neuronal tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sordo L, Indave BI, Barrio G, Degenhardt L, de la Fuente L, Bravo MJ . Cocaine use and risk of stroke: a systematic review. Drug Alcohol Depend 2014; 142: 1–13.
De Giorgi A, Fabbian F, Pala M, Bonetti F, Babini I, Bagnaresi I et al. Cocaine and acute vascular diseases. Curr Drug Abuse Rev 2012; 5: 129–134.
Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K . Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry 1988; 152: 641–648.
Tumeh SS, Nagel JS, English RJ, Moore M, Holman BL . Cerebral abnormalities in cocaine abusers: demonstration by SPECT perfusion brain scintigraphy. Work in progress. Radiology 1990; 176: 821–824.
Mena I, Giombetti RJ, Miller BL, Garrett K, Villanueva-Meyer J, Mody C et al. Cerebral blood flow changes with acute cocaine intoxication: clinical correlations with SPECT, CT, and MRI. NIDA Res Monogr 1994; 138: 161–173.
Kosten TR, Cheeves C, Palumbo J, Seibyl JP, Price LH, Woods SW . Regional cerebral blood flow during acute and chronic abstinence from combined cocaine-alcohol abuse. Drug Alcohol Depend 1998; 50: 187–195.
Ernst T, Chang L, Oropilla G, Gustavson A, Speck O . Cerebral perfusion abnormalities in abstinent cocaine abusers: a perfusion MRI and SPECT study. Psychiatry Res 2000; 99: 63–74.
Kaufman MJ, Levin JM, Ross MH, Lange N, Rose SL, Kukes TJ et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 1998; 279: 376–380.
Volkow ND, Ding YS, Fowler JS, Wang GJ . Cocaine addiction: hypothesis derived from imaging studies with PET. J Addict Dis 1996; 15: 55–71.
Martin PJ, Evans DH, Naylor AR . Transcranial color-coded sonography of the basal cerebral circulation. Reference data from 115 volunteers. Stroke 1994; 25: 390–396.
Cho SJ, Sohn YH, Kim GW, Kim JS . Blood flow velocity changes in the middle cerebral artery as an index of the chronicity of hypertension. J Neurol Sci 1997; 150: 77–80.
Schondorf R, Benoit J, Wein T . Cerebrovascular and cardiovascular measurements during neurally mediated syncope induced by head-up tilt. Stroke 1997; 28: 1564–1568.
Kaufman MJ, Levin JM, Maas LC, Rose SL, Lukas SE, Mendelson JH et al. Cocaine decreases relative cerebral blood volume in humans: a dynamic susceptibility contrast magnetic resonance imaging study. Psychopharmacology (Berl) 1998; 138: 76–81.
Du C, Pan Y . Optical detection of brain function: simultaneous imaging of cerebral vascular response, tissue metabolism, and cellular activity in vivo. Rev Neurosci 2011; 22: 695–709.
Mody CK, Miller BL, McIntyre HB, Cobb SK, Goldberg MA . Neurologic complications of cocaine abuse. Neurology 1988; 38: 1189–1193.
Peterson PK, Gekker G, Chao CC, Schut R, Molitor TW, Balfour HH Jr . Cocaine potentiates HIV-1 replication in human peripheral blood mononuclear cell cocultures. Involvement of transforming growth factor-beta. J Immunol 1991; 146: 81–84.
Neiman J, Haapaniemi HM, Hillbom M . Neurological complications of drug abuse: pathophysiological mechanisms. Eur J Neurol 2000; 7: 595–606.
Levine SR, Welch KM . Cocaine and stroke. Stroke 1988; 19: 779–783.
Klonoff DC, Andrews BT, Obana WG . Stroke associated with cocaine use. Arch Neurol 1989; 46: 989.
Ren H, Du C, Pan Y . Cerebral blood flow imaged with ultrahigh-resolution optical coherence angiography and Doppler tomography. Opt Lett 2012; 37: 1388–1390.
Pan Y, You J, Volkow ND, Park K, Du C . Ultrasensitive detection of 3D cerebral microvascular network dynamics in vivo. NeuroImage 2014; 103: 492–501.
Li B, Freeman RD . High-resolution neurometabolic coupling in the lateral geniculate nucleus. J Neurosci 2007; 27: 10223–10229.
Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A . Neurophysiological investigation of the basis of the fMRI signal. Nature 2001; 412: 150–157.
Roy CS, Sherrington CS . On the regulation of the blood-supply of the brain. J Physiol 1890; 11: 85–158 117.
Iannetti G, Wise RG . BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging 2007; 25: 978–988.
Gollub RL, Breiter HC, Kantor H, Kennedy D, Gastfriend D, Mathew RT et al. Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects. J Cerebr Blood Flow Metab 1998; 18: 724–734.
Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK et al. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med 2000; 43: 45–51.
Ritz MC, Cone EJ, Kuhar MJ . Cocaine inhibition of ligand-binding at dopamine, norepinephrine and serotonin transporters—a structure–activity study. Life Sci 1990; 46: 635–645.
Rebec GV . Behavioral electrophysiology of psychostimulants. Neuropsychopharmacology 2006; 31: 2341–2348.
Jimenez-Rivera CA, Segarra O, Jimenez Z, Waterhouse BD . Effects of intravenous cocaine administration on cerebellar Purkinje cell activity. Eur J Pharmacol 2000; 407: 91–100.
Ueki M, Mies G, Hossmann KA . Effect of alpha-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand 1992; 36: 318–322.
Masamoto K, Kim T, Fukuda M, Wang P, Kim SG . Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex 2007; 17: 942–950.
Bonvento G, Charbonné R, Corrèze J-L, Borredon J, Seylaz J, Lacombe P . Is α-chloralose plus halothane induction a suitable anesthetic regimen for cerebrovascular research? Brain Res 1994; 665: 213–221.
Benveniste H, Volkow ND . Dopamine-enhancing medications to accelerate emergence from general anesthesia. Anesthesiology 2013; 118: 5–6.
Gozzi A, Ceolin L, Schwarz A, Reese T, Bertani S, Crestan V et al. A multimodality investigation of cerebral hemodynamics and autoregulation in pharmacological MRI. Magn Reson Imaging 2007; 25: 826–833.
Yuan Z, Luo Z, Volkow ND, Pan Y, Du C . Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. NeuroImage 2011; 54: 1130–1139.
Wallace DR, Mactutus CF, Booze RM . Repeated intravenous cocaine administration: locomotor activity and dopamine D2/D3 receptors. Synapse 1996; 23: 152–163.
Wang G-J, Volkow ND, Fowler JS, Ferrieri R, Schlyer DJ, Alexoff D et al. Methylphenidate decreases regional cerebral blood flow in normal human subjects. Life Sci 1994; 54: PL143–PL146.
London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM et al. Cocaine-induced reduction of glucose utilization in human brain: a study using positron emission tomography and [Fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry 1990; 47: 567–574.
Lee J-H, Telang FW, Springer CS Jr, Volkow ND . Abnormal brain activation to visual stimulation in cocaine abusers. Life Sci 2003; 73: 1953–1961.
Hillman EM . Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci 2014; 37: 161–181.
Du C, Volkow ND, Koretsky AP, Pan Y . Low-frequency calcium oscillations accompany deoxyhemoglobin oscillations in rat somatosensory cortex. Proc Natl Acad Sci USA 2014; 111: E4677–E4686.
Sordo L, Indave B, Barrio G, Degenhardt L, de la Fuente L, Bravo M . Cocaine use and risk of stroke: a systematic review. Drug Alcohol Depend 2014; 142: 1–13.
Ren H, Du C, Yuan Z, Park K, Volkow ND, Pan Y . Cocaine-induced cortical microischemia in the rodent brain: clinical implications. Mol Psychiatry 2012; 17: 1017–1025.
Ueki M, Mies G, Hossmann KA . Effect of alpha‐chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand 1992; 36: 318–322.
Bonvento G, Charbonne R, Correze JL, Borredon J, Seylaz J, Lacombe P . Is alpha-chloralose plus halothane induction a suitable anesthetic regimen for cerebrovascular research. Brain Res 1994; 665: 213–221.
Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA et al. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci USA 2007; 104: 18265–18269.
Schulz K, Sydekum E, Krueppel R, Engelbrecht CJ, Schlegel F, Schröter A et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat Methods 2012; 9: 597–602.
Ma H, Harris S, Rahmani R, Lacefield CO, Zhao M, Daniel AG et al. Wide-field in vivo neocortical calcium dye imaging using a convection-enhanced loading technique combined with simultaneous multiwavelength imaging of voltage-sensitive dyes and hemodynamic signals. Neurophotonics 2014; 1: 015003–015003.
Moxon K, Hale L, Aguilar J, Foffani G . Responses of infragranular neurons in the rat primary somatosensory cortex to forepaw and hindpaw tactile stimuli. Neuroscience 2008; 156: 1083–1092.
Acknowledgements
This work was supported, in part, by National Institutes of Health (NIH) Grants R21DA032228 (YP/CD), 1R01DA029718 (CD/YP), R01NS084817 (CD) and NIH’s Intramural Program (NDV).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, W., Liu, P., Volkow, N. et al. Cocaine attenuates blood flow but not neuronal responses to stimulation while preserving neurovascular coupling for resting brain activity. Mol Psychiatry 21, 1408–1416 (2016). https://doi.org/10.1038/mp.2015.185
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.185
This article is cited by
-
Cocaine’s cerebrovascular vasoconstriction is associated with astrocytic Ca2+ increase in mice
Communications Biology (2022)
-
Ca2+ channel blockade reduces cocaine’s vasoconstriction and neurotoxicity in the prefrontal cortex
Translational Psychiatry (2021)
-
Suppression of neuropathic pain and comorbidities by recurrent cycles of repetitive transcranial direct current motor cortex stimulation in mice
Scientific Reports (2021)
-
Cocaine-induced ischemia in prefrontal cortex is associated with escalation of cocaine intake in rodents
Molecular Psychiatry (2020)
-
Cocaine addicted rats show reduced neural activity as revealed by manganese-enhanced MRI
Scientific Reports (2020)