Abstract
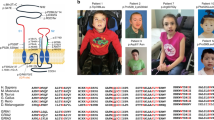
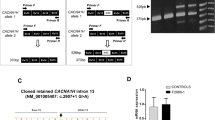
Autism spectrum disorder (ASD) is a common neurodevelopmental condition characterized by marked genetic heterogeneity. Recent studies of rare structural and sequence variants have identified hundreds of loci involved in ASD, but our knowledge of the overall genetic architecture and the underlying pathophysiological mechanisms remains incomplete. Glycine receptors (GlyRs) are ligand-gated chloride channels that mediate inhibitory neurotransmission in the adult nervous system but exert an excitatory action in immature neurons. GlyRs containing the α2 subunit are highly expressed in the embryonic brain, where they promote cortical interneuron migration and the generation of excitatory projection neurons. We previously identified a rare microdeletion of the X-linked gene GLRA2, encoding the GlyR α2 subunit, in a boy with autism. The microdeletion removes the terminal exons of the gene (GLRA2Δex8–9). Here, we sequenced 400 males with ASD and identified one de novo missense mutation, p.R153Q, absent from controls. In vitro functional analysis demonstrated that the GLRA2Δex8–9 protein failed to localize to the cell membrane, while the R153Q mutation impaired surface expression and markedly reduced sensitivity to glycine. Very recently, an additional de novo missense mutation (p.N136S) was reported in a boy with ASD, and we show that this mutation also reduced cell-surface expression and glycine sensitivity. Targeted glra2 knockdown in zebrafish induced severe axon-branching defects, rescued by injection of wild type but not GLRA2Δex8–9 or R153Q transcripts, providing further evidence for their loss-of-function effect. Glra2 knockout mice exhibited deficits in object recognition memory and impaired long-term potentiation in the prefrontal cortex. Taken together, these results implicate GLRA2 in non-syndromic ASD, unveil a novel role for GLRA2 in synaptic plasticity and learning and memory, and link altered glycinergic signaling to social and cognitive impairments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 2011; 70: 863–885.
Betancur C . Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res 2011; 1380: 42–77.
Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet 2014; 94: 677–694.
De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Kou Y et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014; 515: 209–215.
Iossifov I, O'Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014; 515: 216–221.
Lubs HA, Stevenson RE, Schwartz CE . Fragile X and X-linked intellectual disability: four decades of discovery. Am J Hum Genet 2012; 90: 579–590.
Ebert DH, Greenberg ME . Activity-dependent neuronal signalling and autism spectrum disorder. Nature 2013; 493: 327–337.
Lynch JW . Native glycine receptor subtypes and their physiological roles. Neuropharmacology 2009; 56: 303–309.
Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H . Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J 1991; 10: 2401–2409.
Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M et al. Spatio-temporal transcriptome of the human brain. Nature 2011; 478: 483–489.
Thomas RH, Chung SK, Wood SE, Cushion TD, Drew CJ, Hammond CL et al. Genotype-phenotype correlations in hyperekplexia: apnoeas, learning difficulties and speech delay. Brain 2013; 136: 3085–3095.
Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H et al. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 2004; 304: 884–887.
Winkelmann A, Maggio N, Eller J, Caliskan G, Semtner M, Haussler U et al. Changes in neural network homeostasis trigger neuropsychiatric symptoms. J Clin Invest 2014; 124: 696–711.
Flint AC, Liu X, Kriegstein AR . Nonsynaptic glycine receptor activation during early neocortical development. Neuron 1998; 20: 43–53.
Ben-Ari Y . Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 2002; 3: 728–739.
Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L . Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron 2002; 36: 1051–1061.
Scain AL, Le Corronc H, Allain AE, Muller E, Rigo JM, Meyrand P et al. Glycine release from radial cells modulates the spontaneous activity and its propagation during early spinal cord development. J Neurosci 2010; 30: 390–403.
Spitzer NC . Electrical activity in early neuronal development. Nature 2006; 444: 707–712.
LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR . GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 1995; 15: 1287–1298.
Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L et al. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci 2005; 25: 4755–4765.
Bortone D, Polleux F . KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron 2009; 62: 53–71.
Sanes DH, Chokshi P . Glycinergic transmission influences the development of dendrite shape. Neuroreport 1992; 3: 323–326.
Furuya S, Tabata T, Mitoma J, Yamada K, Yamasaki M, Makino A et al. L-serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons. Proc Natl Acad Sci USA 2000; 97: 11528–11533.
Tapia JC, Mentis GZ, Navarrete R, Nualart F, Figueroa E, Sanchez A et al. Early expression of glycine and GABAA receptors in developing spinal cord neurons. Effects on neurite outgrowth. Neuroscience 2001; 108: 493–506.
Avila A, Vidal PM, Dear TN, Harvey RJ, Rigo JM, Nguyen L . Glycine receptor α2 subunit activation promotes cortical interneuron migration. Cell Rep 2013; 4: 738–750.
Avila A, Vidal PM, Tielens S, Morelli G, Laguesse S, Harvey RJ et al. Glycine receptors control the generation of projection neurons in the developing cerebral cortex. Cell Death Differ 2014; 21: 1696–1708.
Young TL, Cepko CL . A role for ligand-gated ion channels in rod photoreceptor development. Neuron 2004; 41: 867–879.
Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010; 466: 368–372.
Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 2007; 39: 25–27.
Gillberg C, Gillberg C, Rastam M, Wentz E . The Asperger Syndrome (and high-functioning autism) Diagnostic Interview (ASDI): a preliminary study of a new structured clinical interview. Autism 2001; 5: 57–66.
Soding J, Biegert A, Lupas AN . The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res 2005; 33: W244–W248.
Pless SA, Hanek AP, Price KL, Lynch JW, Lester HA, Dougherty DA et al. A cation-pi interaction at a phenylalanine residue in the glycine receptor binding site is conserved for different agonists. Mol Pharmacol 2011; 79: 742–748.
Bai J, Blot K, Tzavara E, Nosten-Bertrand M, Giros B, Otani S . Inhibition of dopamine transporter activity impairs synaptic depression in rat prefrontal cortex through over-stimulation of D1 receptors. Cereb Cortex 2014; 24: 945–955.
Yuen RK, Thiruvahindrapuram B, Merico D, Walker S, Tammimies K, Hoang N et al. Whole-genome sequencing of quartet families with autism spectrum disorder. Nat Med 2015; 21: 185–191.
Piton A, Gauthier J, Hamdan FF, Lafreniere RG, Yang Y, Henrion E et al. Systematic resequencing of X-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol Psychiatry 2011; 16: 867–880.
Villmann C, Oertel J, Melzer N, Becker CM . Recessive hyperekplexia mutations of the glycine receptor alpha1 subunit affect cell surface integration and stability. J Neurochem 2009; 111: 837–847.
Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA . fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex 2008; 18: 289–300.
Sahyoun CP, Belliveau JW, Soulieres I, Schwartz S, Mody M . Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia 2010; 48: 86–95.
Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med 2014; 370: 1209–1219.
Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H et al. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 2005; 45: 727–739.
Todorovic J, Welsh BT, Bertaccini EJ, Trudell JR, Mihic SJ . Disruption of an intersubunit electrostatic bond is a critical step in glycine receptor activation. Proc Natl Acad Sci USA 2010; 107: 7987–7992.
Bode A, Lynch JW . The impact of human hyperekplexia mutations on glycine receptor structure and function. Mol Brain 2014; 7: 2.
Kabashi E, Champagne N, Brustein E, Drapeau P . In the swim of things: recent insights to neurogenetic disorders from zebrafish. Trends Genet 2010; 26: 373–381.
Warburton EC, Brown MW . Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia 2010; 48: 2262–2272.
Brown MW, Barker GR, Aggleton JP, Warburton EC . What pharmacological interventions indicate concerning the role of the perirhinal cortex in recognition memory. Neuropsychologia 2012; 50: 3122–3140.
Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW Jr . The rodent hippocampus is essential for nonspatial object memory. Curr Biol 2013; 23: 1685–1690.
Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D et al. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem 2007; 14: 117–125.
Rinaldi A, Romeo S, Agustin-Pavon C, Oliverio A, Mele A . Distinct patterns of Fos immunoreactivity in striatum and hippocampus induced by different kinds of novelty in mice. Neurobiol Learn Mem 2010; 94: 373–381.
Barbosa FF, Santos JR, Meurer YS, Macedo PT, Ferreira LM, Pontes IM et al. Differential cortical c-Fos and Zif-268 expression after object and spatial memory processing in a standard or episodic-like object recognition task. Front Behav Neurosci 2013; 7: 112.
Tanaka DH, Toriumi K, Kubo K, Nabeshima T, Nakajima K . GABAergic precursor transplantation into the prefrontal cortex prevents phencyclidine-induced cognitive deficits. J Neurosci 2011; 31: 14116–14125.
Fan J . Attentional network deficits in autism spectrum disorders. In: Buxbaum JD, Hof PR (eds). The Emerging Neuroscience of Autism Spectrum Disorders. Elsevier: : Oxford, UK, 2011, pp 281–288.
Marin O . Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 2012; 13: 107–120.
Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 2007; 318: 71–76.
Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 2010; 468: 263–269.
Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 2011; 147: 235–246.
Paluszkiewicz SM, Martin BS, Huntsman MM . Fragile × syndrome: the GABAergic system and circuit dysfunction. Dev Neurosci 2011; 33: 349–364.
Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB et al. Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature 2012; 489: 385–390.
Rubenstein JL, Merzenich MM . Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2003; 2: 255–267.
Rubenstein JL . Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Curr Opin Neurol 2010; 23: 118–123.
Gogolla N, Leblanc JJ, Quast KB, Sudhof TC, Fagiolini M, Hensch TK . Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord 2009; 1: 172–181.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011; 477: 171–178.
Sudhof TC . Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008; 455: 903–911.
Vafa B, Lewis TM, Cunningham AM, Jacques P, Lynch JW, Schofield PR . Identification of a new ligand binding domain in the alpha1 subunit of the inhibitory glycine receptor. J Neurochem 1999; 73: 2158–2166.
Acknowledgements
We are grateful to the families for their participation. We thank Marika Nosten-Bertrand and Stéphanie Daumas for advice on behavioral analyses, Joseph D Buxbaum for helpful discussions, Laïla Gasmi for technical help, Guillaume Pezeron and Isabelle Anselme for help with zebrafish in situ hybridization experiments, Annie Munier for technical support at the Flow Cytometry Facility of the Saint-Antoine Research Center (UPMC), the Institute of Biology Paris-Seine Imaging facility, the DNA and cell bank of the Pitié-Salpêtrière Hospital and the Clinical Investigation Center of the Robert Debré Hospital. This work was supported by a NARSAD Independent Investigator Award from the Brain & Behavior Research Foundation to CB, the Foundation for Autism Research, INSERM, CNRS and UPMC. GN and CG were supported by the Swedish Science Council and by the Annmari and Per Ahlqvist Foundation, RJH and TND by the Medical Research Council (G0500833), JCM by the Bundesministerium für Bildung und Forschung BMBF (Era-Net NEURON II CIPRESS), and MT by a Medical Research Council Centenary Award (G0600084), BBSRC (BB/K01692X/1) and the Leverhulme Trust (RPG-2012-519). MP and ED were supported by PhD fellowships from the French Ministry of Research. We gratefully acknowledge the CNV resources provided by the Autism Genome Project consortium, funded by Autism Speaks, the Health Research Board of Ireland, the Medical Research Council, Genome Canada/Ontario Genomics Institute and the Hilibrand Foundation.
Author contributions
MP and CB conceived and designed the study; MP and AP performed sequencing experiments and variant confirmation; ED participated in the genetic analyses; BA, VG, FD, GN, MR, RD, CG and ML participated in subject recruitment and assessment; JCM provided critical reagents and advice; MP and SG performed site-directed mutagenesis; MP and HLC performed the immunohistochemistry; HLC and PL performed the in vitro electrophysiology studies; SDG performed the biotinylation experiments; VMJ, MT and RJH performed molecular modeling; MP, CF and JH performed the zebrafish experiments; TND and RJH generated Glra2 knockout mice; MP performed the behavioral experiments and analyzed the results with CB; HC contributed to mouse breeding and behavioral analyses; JB and SO performed the ex vivo electrophysiology studies; BG provided key facilities and equipment and consulted on the execution and interpretation of the behavioral and electrophysiological studies; CB coordinated the study; MP and CB wrote the manuscript. All authors reviewed and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Pilorge, M., Fassier, C., Le Corronc, H. et al. Genetic and functional analyses demonstrate a role for abnormal glycinergic signaling in autism. Mol Psychiatry 21, 936–945 (2016). https://doi.org/10.1038/mp.2015.139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.139
This article is cited by
-
AAV-glycine receptor α3 alleviates CFA-induced inflammatory pain by downregulating ERK phosphorylation and proinflammatory cytokine expression in SD rats
Molecular Medicine (2023)
-
Whole exome sequencing revealed variants in four genes underlying X-linked intellectual disability in four Iranian families: novel deleterious variants and clinical features with the review of literature
BMC Medical Genomics (2023)
-
Comprehensive behavioral analyses of mice with a glycine receptor alpha 4 deficiency
Molecular Brain (2023)
-
Computational prognostic evaluation of Alzheimer’s drugs from FDA-approved database through structural conformational dynamics and drug repositioning approaches
Scientific Reports (2023)
-
Cannabidiol for the treatment of autism spectrum disorder: hope or hype?
Psychopharmacology (2022)