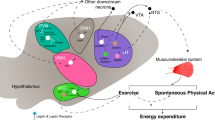
Abstract
The brain receives and integrates environmental and metabolic information, transforms these signals into adequate neuronal circuit activities, and generates physiological behaviors to promote energy homeostasis. The responsible neuronal circuitries show lifetime plasticity and guaranty metabolic health and survival. However, this highly evolved organization has become challenged nowadays by chronic overload with nutrients and reduced physical activity, which results in an ever-increasing number of obese individuals worldwide. Research within the last two decades has aimed to decipher the responsible molecular and cellular mechanisms for regulation of the hypothalamic melanocortin neurons, which have a key role in the control of food intake and energy metabolism. This review maps the central connections of the melanocortin system and highlights its global position and divergent character in physiological and pathological metabolic events. Moreover, recently uncovered molecular and cellular processes in hypothalamic neurons and glial cells that drive plastic morphological and physiological changes in these cells, and account for regulation of food intake and energy metabolism, are brought into focus. Finally, potential functional interactions between metabolic disorders and psychiatric diseases are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dietrich MO, Horvath TL . Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci 2013; 36: 65–73.
Zeltser LM, Seeley RJ, Tschop MH . Synaptic plasticity in neuronal circuits regulating energy balance. Nat Neurosci 2012; 15: 1336–1342.
Ford ES, Giles WH, Dietz WH . Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002; 287: 356–359.
Mattson MP . Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab 2012; 16: 706–722.
Kaidanovich-Beilin O, Cha DS, McIntyre RS . Crosstalk between metabolic and neuropsychiatric disorders. F1000 Biol Rep 2012; 4: 14.
Girault EM, Yi CX, Fliers E, Kalsbeek A . Orexins feeding, and energy balance. Prog Brain Res 2012; 198: 47–64.
DiLeone RJ, Taylor JR, Picciotto MR . The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci 2012; 15: 1330–1335.
Grill HJ, Hayes MR . Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 2012; 16: 296–309.
Morton GJ, Schwartz MW . Leptin and the central nervous system control of glucose metabolism. Physiol Rev 2011; 91: 389–411.
Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 2007; 117: 3475–3488.
Pelletier G, Dube D . Electron microscopic immunohistochemical localization of alpha-MSH in the rat brain. Am J Anat 1977; 150: 201–205.
Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD . Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 1997; 385: 165–168.
Hahn TM, Breininger JF, Baskin DG, Schwartz MW . Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1998; 1: 271–272.
Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C . Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res 1997; 756: 283–286.
Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001; 411: 480–484.
Wu Q, Boyle MP, Palmiter RD . Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 2009; 137: 1225–1234.
Dietrich MO, Horvath TL . GABA keeps up an appetite for life. Cell 2009; 137: 1177–1179.
Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I et al. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 2007; 13: 89–94.
Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 2004; 304: 110–115.
Horvath TL, Diano S . The floating blueprint of hypothalamic feeding circuits. Nat Rev Neurosci 2004; 5: 662–667.
Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature 2008; 454: 846–851.
Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem 1993; 268: 15174–15179.
Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD . Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 1994; 8: 1298–1308.
Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997; 88: 131–141.
Vaisse C, Clement K, Guy-Grand B, Froguel P . A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet 1998; 20: 113–114.
Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 2013; 341: 275–278.
Sebag JA, Zhang C, Hinkle PM, Bradshaw AM, Cone RD . Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science 2013; 341: 278–281.
Liu T, Elmquist JK, Williams KW . Mrap2: an accessory protein linked to obesity. Cell Metab 2013; 18: 309–311.
Williams KW, Elmquist JK . From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci 2012; 15: 1350–1355.
Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005; 123: 493–505.
Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 2011; 13: 195–204.
Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB et al. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 2013; 152: 612–619.
Yaswen L, Diehl N, Brennan MB, Hochgeschwender U . Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 1999; 5: 1066–1070.
Xu Y, Elmquist JK, Fukuda M . Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann N Y Acad Sci 2011; 1243: 1–14.
Erickson JC, Clegg KE, Palmiter RD . Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 1996; 381: 415–421.
Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol 2002; 22: 5027–5035.
Wu Q, Howell MP, Cowley MA, Palmiter RD . Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc Natl Acad Sci USA 2008; 105: 2687–2692.
Carter ME, Soden ME, Zweifel LS, Palmiter RD . Genetic identification of a neural circuit that suppresses appetite. Nature 2013; 503: 111–114.
Wu Q, Clark MS, Palmiter RD . Deciphering a neuronal circuit that mediates appetite. Nature 2012; 483: 594–597.
Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB . Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 2008; 11: 998–1000.
Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 2013; 33: 3624–3632.
Aponte Y, Atasoy D, Sternson SM . AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 2011; 14: 351–355.
Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 2011; 121: 1424–1428.
Atasoy D, Betley JN, Su HH, Sternson SM . Deconstruction of a neural circuit for hunger. Nature 2012; 488: 172–177.
Krashes MJ, Shah BP, Koda S, Lowell BB . Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab 2013; 18: 588–595.
Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 2007; 5: 438–449.
Horvath TL, Andrews ZB, Diano S . Fuel utilization by hypothalamic neurons: roles for ROS. Trends Endocrinol Metab 2009; 20: 78–87.
Grayson BE, Seeley RJ, Sandoval DA . Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci 2013; 14: 24–37.
Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 2007; 449: 228–232.
Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV . Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes 1998; 47: 294–297.
Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 2010; 11: 286–297.
Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003; 37: 649–661.
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004; 428: 569–574.
Steinberg GR, Kemp BE . AMPK in health and disease. Physiol Rev 2009; 89: 1025–1078.
Kola B . Role of AMP-activated protein kinase in the control of appetite. J Neuroendocrinol 2008; 20: 942–951.
Chari M Lam CKL Lam TKT . Hypothalamic fatty acid sensing in the normal and disease states. In: Montmayeur JP, Le Coutre J (eds). Fat Detection: Taste, Texture, and Post Ingestive Effects. CRC Press: Boca Raton, FL, USA (2010).
Diano S, Horvath L . Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med 2012; 18: 52–58.
Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, Kim E et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med 2011; 17: 1121–1127.
Bonnefont-Rousselot D . Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care 2002; 5: 561–568.
Yang Y, Atasoy D, Su HH, Sternson SM . Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 2011; 146: 992–1003.
Norsted E, Gomuc B, Meister B . Protein components of the blood-brain barrier (BBB) in the mediobasal hypothalamus. J Chem Neuroanat 2008; 36: 107–121.
Faouzi M, Leshan R, Bjornholm M, Hennessey T, Jones J, Munzberg H . Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology 2007; 148: 5414–5423.
Levin BE, Magnan C, Dunn-Meynell A, Le Foll C . Metabolic sensing and the brain: who, what, where, and how? Endocrinology 2011; 152: 2552–2557.
Prevot V, Langlet F, Dehouck B . Flipping the tanycyte switch: how circulating signals gain direct access to the metabolic brain. Aging 2013; 5: 332–334.
Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab 2013; 17: 607–617.
Myers MG Jr . How is the hungry brain like a sieve? Cell Metab 2013; 17: 467–468.
Bolborea M, Dale N . Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci 2013; 36: 91–100.
Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci 2012; 15: 700–702.
Dietrich MO, Horvath TL . Fat incites tanycytes to neurogenesis. Nat Neurosci 2012; 15: 651–653.
Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 2007; 5: 181–194.
Yi CX, Gericke M, Kruger M, Alkemade A, Kabra DG, Hanske S et al. High calorie diet triggers hypothalamic angiopathy. Mol Metab 2012; 1: 95–100.
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012; 122: 153–162.
Garcia-Caceres C, Yi CX, Tschop MH . Hypothalamic astrocytes in obesity. Endocrinol Metab Clin North Am 2013; 42: 57–66.
Vogt MC, Paeger L, Hess S, Steculorum SM, Awazawa M, Hampel B et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 2014; 156: 495–509.
Garcia-Segura LM, Baetens D, Naftolin F . Synaptic remodelling in arcuate nucleus after injection of estradiol valerate in adult female rats. Brain Res 1986; 366: 131–136.
Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA 2010; 107: 14875–14880.
Fuente-Martin E, Garcia-Caceres C, Granado M, de Ceballos ML, Sanchez-Garrido MA, Sarman B et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest 2012; 122: 3900–3913.
Shen L, Tso P, Wang DQ, Woods SC, Davidson WS, Sakai R et al. Up-regulation of apolipoprotein E by leptin in the hypothalamus of mice and rats. Physiol Behav 2009; 98: 223–228.
Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL . Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 2010; 151: 1622–1632.
Tapia-Gonzalez S, Garcia-Segura LM, Tena-Sempere M, Frago LM, Castellano JM, Fuente-Martin E et al. Activation of microglia in specific hypothalamic nuclei and the cerebellum of adult rats exposed to neonatal overnutrition. J Neuroendocrinol 2011; 23: 365–370.
Lafrance V, Inoue W, Kan B, Luheshi GN . Leptin modulates cell morphology and cytokine release in microglia. Brain Behav Immun 2010; 24: 358–365.
Bulgarelli I, Tamiazzo L, Bresciani E, Rapetti D, Caporali S, Lattuada D et al. Desacyl-ghrelin and synthetic GH-secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by beta-amyloid fibrils. J Neurosci Res 2009; 87: 2718–2727.
Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, Schwartz MW . Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes 2013; 62: 2629–2634.
Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC et al. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain 2009; 132: 889–902.
Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 2004; 10: 739–743.
Mohanty A, McBride HM . Emerging roles of mitochondria in the evolution, biogenesis, and function of peroxisomes. Front Physiol 2013; 4: 268.
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306: 457–461.
Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009; 9: 35–51.
Dietrich MO, Liu ZW, Horvath TL . Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell 2013; 155: 188–199.
Zorzano A, Sebastian D, Segales J, Palacin M . The molecular machinery of mitochondrial fusion and fission: an opportunity for drug discovery? Curr Opin Drug Discov Dev 2009; 12: 597–606.
Schneeberger M, Dietrich MO, Sebastian D, Imbernon M, Castano C, Garcia A et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 2013; 155: 172–187.
Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO et al. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat Neurosci 2012; 15: 1108–1110.
Palmiter RD . New game for hunger neurons. Nat Neurosci 2012; 15: 1060–1061.
Sarrar L, Ehrlich S, Merle JV, Pfeiffer E, Lehmkuhl U, Schneider N . Cognitive flexibility and agouti-related protein in adolescent patients with anorexia nervosa. Psychoneuroendocrinology 2011; 36: 1396–1406.
Tsankova N, Renthal W, Kumar A, Nestler EJ . Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci 2007; 8: 355–367.
Rutter M . Achievements and challenges in the biology of environmental effects. Proc Natl Acad Sci USA 2012; 109: 17149–17153.
Megna JL, Schwartz TL, Siddiqui UA, Herrera Rojas M . Obesity in adults with serious and persistent mental illness: a review of postulated mechanisms and current interventions. Ann Clin Psychiatry 2011; 23: 131–140.
Torrent C, Amann B, Sanchez-Moreno J, Colom F, Reinares M, Comes M et al. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand 2008; 118: 4–18.
Jones M, Jones A . The effect of antipsychotic medication on metabolic syndrome. Nursing Standard 2008; 22: 43–48.
Jow GM, Yang TT, Chen CL . Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord 2006; 90: 21–27.
Raikkonen K, Matthews KA, Kuller LH . Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care 2007; 30: 872–877.
St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA 2005; 294: 557–562.
Altschuler EL . Schizophrenia and the Chinese famine of 1959-1961. JAMA 2005; 294: 2968, discussion 2968-2969.
Neugebauer R . Accumulating evidence for prenatal nutritional origins of mental disorders. JAMA 2005; 294: 621–623.
Chandler-Laney PC, Castaneda E, Pritchett CE, Smith ML, Giddings M, Artiga AI et al. A history of caloric restriction induces neurochemical and behavioral changes in rats consistent with models of depression. Pharmacol Biochem Behav 2007; 87: 104–114.
Ikegami M, Ikeda H, Ishikawa Y, Ohsawa M, Ohashi T, Kai M et al. Olanzapine induces glucose intolerance through the activation of AMPK in the mouse hypothalamus. Eur J Pharmacol 2013; 718: 376–382.
Weston-Green K, Huang XF, Deng C . Alterations to melanocortinergic, GABAergic and cannabinoid neurotransmission associated with olanzapine-induced weight gain. PloS One 2012; 7: e33548.
Eder U, Mangweth B, Ebenbichler C, Weiss E, Hofer A, Hummer M et al. Association of olanzapine-induced weight gain with an increase in body fat. Am J Psychiatry 2001; 158: 1719–1722.
Pannacciulli N, Vettor R, Milan G, Granzotto M, Catucci A, Federspil G et al. Anorexia nervosa is characterized by increased adiponectin plasma levels and reduced nonoxidative glucose metabolism. J Clin Endocrinol Metab 2003; 88: 1748–1752.
Lustman PJ, Clouse RE . Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications 2005; 19: 113–122.
Lustman PJ, Clouse RE . Depression in diabetes: the chicken or the egg? Psychosom Med 2007; 69: 297–299.
Dietrich MO, Horvath TL . Limitations in anti-obesity drug development: the critical role of hunger-promoting neurons. Nat Rev Drug Discov 2012; 11: 675–691.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Koch, M., Horvath, T. Molecular and cellular regulation of hypothalamic melanocortin neurons controlling food intake and energy metabolism. Mol Psychiatry 19, 752–761 (2014). https://doi.org/10.1038/mp.2014.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.30
This article is cited by
-
Bitter taste cells in the ventricular walls of the murine brain regulate glucose homeostasis
Nature Communications (2023)
-
Silencing of hypothalamic FGF11 prevents diet-induced obesity
Molecular Brain (2022)
-
GPCR and Alcohol-Related Behaviors in Genetically Modified Mice
Neurotherapeutics (2020)
-
Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism
Nature Neuroscience (2019)
-
Functional identity of hypothalamic melanocortin neurons depends on Tbx3
Nature Metabolism (2019)