Abstract
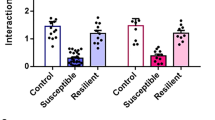
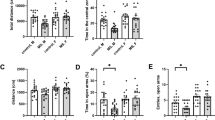
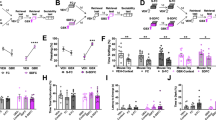
The Abelson helper integration site 1 (AHI1) gene has a pivotal role in brain development. Studies by our group and others have demonstrated association of AHI1 with schizophrenia and autism. To elucidate the mechanism whereby alteration in AHI1 expression may be implicated in the pathogenesis of neuropsychiatric disorders, we studied Ahi1 heterozygous knockout (Ahi1+/−) mice. Although their performance was not different from wild-type mice on tests that model classical schizophrenia-related endophenotypes, Ahi1+/− mice displayed an anxiolytic-like phenotype across different converging modalities. Using behavioral paradigms that involve exposure to environmental and social stress, significantly decreased anxiety was evident in the open field, elevated plus maze and dark–light box, as well as during social interaction in pairs. Assessment of core temperature and corticosterone secretion revealed a significantly blunted response of the autonomic nervous system and the hypothalamic–pituitary–adrenal axis in Ahi1+/− mice exposed to environmental and visceral stress. However, response to centrally acting anxiogenic compounds was intact. On resting-state functional MRI, connectivity of the amygdala with other brain regions involved in processing of anxiogenic stimuli and inhibitory avoidance learning, such as the lateral entorhinal cortex, ventral hippocampus and ventral tegmental area, was significantly reduced in the mutant mice. Taken together, our data link Ahi1 under-expression with a defect in the process of threat detection. Alternatively, the results could be interpreted as representing an anxiety-related endophenotype, possibly granting the Ahi1+/− mouse relative resilience to various types of stress. The current knockout model highlights the contribution of translational approaches to understanding the genetic basis of emotional regulation and its associated neurocircuitry, with possible relevance to neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Geschwind DH . Autism: many genes, common pathways? Cell 2008; 135: 391–395.
Rapoport JL, Addington AM, Frangou S, Psych MR . The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry 2005; 10: 434–449.
Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol 2008; 20: 1297–1327.
Minshew NJ, Williams DL . The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol 2007; 64: 945–950.
Kas MJ, Fernandes C, Schalkwyk LC, Collier DA . Genetics of behavioural domains across the neuropsychiatric spectrum; of mice and men. Mol Psychiatry 2007; 12: 324–330.
Mitchell KJ . The genetics of neurodevelopmental disease. Curr Opin Neurobiol 2011; 21: 197–203.
Sebat J, Levy DL, McCarthy SE . Rare structural variants in schizophrenia: one disorder, multiple mutations; one mutation, multiple disorders. Trends Genet 2009; 25: 528–535.
Dixon-Salazar T, Silhavy J, Marsh S, Louie C, Scott L, Gururaj A et al. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet 2004; 75: 979–987.
Ferland R, Eyaid W, Collura R, Tully L, Hill R, Al-Nouri D et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet 2004; 36: 1008–1013.
Jiang X, Hanna Z, Kaouass M, Girard L, Jolicoeur P . Ahi-1, a novel gene encoding a modular protein with WD40-repeat and SH3 domains, is targeted by the Ahi-1 and Mis-2 provirus integrations. J Virol 2002; 76: 9046–9059.
Yu F, Keinan A, Chen H, Ferland R, Hill R, Mignault A et al. Detecting natural selection by empirical comparison to random regions of the genome. Hum Mol Genet 2009; 18: 4853–4867.
Doering J, Kane K, Hsiao Y, Yao C, Shi B, Slowik A et al. Species differences in the expression of Ahi1, a protein implicated in the neurodevelopmental disorder Joubert syndrome, with preferential accumulation to stigmoid bodies. J Comp Neurol 2008; 511: 238–256.
Cheng YZ, Eley L, Hynes AM, Overman LM, Simms RJ, Barker A et al. Investigating embryonic expression patterns and evolution of AHI1 and CEP290 genes, implicated in Joubert syndrome. PLoS ONE 2012; 7: e44975.
Doherty D . Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin Pediatr Neurol 2009; 16: 143–154.
Lancaster M, Louie C, Silhavy J, Sintasath L, Decambre M, Nigam S et al. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med 2009; 15: 1046–1054.
Sheng G, Xu X, Lin Y, Wang C, Rong J, Cheng D et al. Huntingtin-associated protein 1 interacts with Ahi1 to regulate cerebellar and brainstem development in mice. J Clin Invest 2008; 118: 2785–2795.
Lancaster MA, Gopal DJ, Kim J, Saleem SN, Silhavy JL, Louie CM et al. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat Med 2011; 17: 726–731.
Westfall JE, Hoyt C, Liu Q, Hsiao YC, Pierce EA, Page-McCaw PS et al. Retinal degeneration and failure of photoreceptor outer segment formation in mice with targeted deletion of the Joubert syndrome gene, Ahi1. J Neurosci 2010; 30: 8759–8768.
Louie C, Caridi G, Lopes V, Brancati F, Kispert A, Lancaster M et al. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet 2010; 42: 175–180.
Lerer B, Segman R, Hamdan A, Kanyas K, Karni O, Kohn Y et al. Genome scan of Arab Israeli families maps a schizophrenia susceptibility gene to chromosome 6q23 and supports a locus at chromosome 10q24. Mol Psychiatry 2003; 8: 488–498.
Amann-Zalcenstein D, Avidan N, Kanyas K, Ebstein R, Kohn Y, Hamdan A et al. AHI1, a pivotal neurodevelopmental gene, and C6orf217 are associated with susceptibility to schizophrenia. Eur J Hum Genet 2006; 14: 1111–1119.
Ingason A, Sigmundsson T, Steinberg S, Sigurdsson E, Haraldsson M, Magnusdottir B et al. Support for involvement of the AHI1 locus in schizophrenia. Eur J Hum Genet 2007; 15: 988–991.
Ingason A, Giegling I, Cichon S, Hansen T, Rasmussen H, Nielsen J et al. A large replication study and meta-analysis in European samples provides further support for association of AHI1 markers with schizophrenia. Hum Mol Genet 2010; 19: 1379–1386.
Rivero O, Reif A, Sanjuán J, Moltó M, Kittel-Schneider S, Nájera C et al. Impact of the AHI1 gene on the vulnerability to schizophrenia: a case-control association study. PLoS ONE 2010; 5: e12254.
Alvarez Retuerto A, Cantor R, Gleeson J, Ustaszewska A, Schackwitz W, Pennacchio L et al. Association of common variants in the Joubert syndrome gene (AHI1) with autism. Hum Mol Genet 2008; 17: 3887–3896.
Allen N, Bagade S, McQueen M, Ioannidis J, Kavvoura F, Khoury M et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet 2008; 40: 827–834.
Xu X, Yang H, Lin YF, Li X, Cape A, Ressler KJ et al. Neuronal Abelson helper integration site-1 (Ahi1) deficiency in mice alters TrkB signaling with a depressive phenotype. Proc Natl Acad Sci USA 2010; 107: 19126–19131.
Weickert CS, Sheedy D, Rothmond DA, Dedova I, Fung S, Garrick T et al. Selection of reference gene expression in a schizophrenia brain cohort. Aust N Z J Psychiatry 2010; 44: 59–70.
Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011; 474: 380–384.
Slonimsky A, Levy I, Kohn Y, Rigbi A, Ben-Asher E, Lancet D et al. Lymphoblast and brain expression of AHI1 and the novel primate-specific gene, C6orf217, in schizophrenia and bipolar disorder. Schizophr Res 2010; 120: 159–166.
Skarnes WC, von Melchner H, Wurst W, Hicks G, Nord AS, Cox T et al. A public gene trap resource for mouse functional genomics. Nat Genet 2004; 36: 543–544.
Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z . Animal model for PTSD: from clinical concept to translational research. Neuropharmacology 2012; 62: 715–724.
Lipina TV, Palomo V, Gil C, Martinez A, Roder JC . Dual inhibitor of PDE7 and GSK-3 - VP1.15 acts as antipsychotic and cognitive enhancer in C57BL/6J mice. Neuropharmacology 2012; 64: 205–214.
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 2004; 3: 287–302.
Griebel G, Belzung C, Misslin R, Vogel E . The free-exploratory paradigm: an effective method for measuring neophobic behaviour in mice and testing potential neophobia-reducing drugs. Behav Pharmacol 1993; 4: 637–644.
Lifschytz T, Broner EC, Zozulinsky P, Slonimsky A, Eitan R, Greenbaum L et al. Relationship between Rgs2 gene expression level and anxiety and depression-like behaviour in a mutant mouse model: serotonergic involvement. Int J Neuropsychopharmacol 2012; 15: 1307–1318.
Adriaan Bouwknecht J, Olivier B, Paylor RE . The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev 2007; 31: 41–59.
Tybout A, Sternthal B, Keppel G, Verducci J, Meyers-Levy J, Barnes J et al. Analysis of variance. J Consumer Psychol 2001; 10: 5–35.
Paxinos G . Atlas of the Developing Mouse Brain at E17.5, P0 and P6 1st (edn) Elsevier: Amsterdam; Boston, 2007.
van den Buuse M . Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull 2010; 36: 246–270.
Powell SB, Weber M, Geyer MA . Genetic models of sensorimotor gating: relevance to neuropsychiatric disorders. Curr Top Behav Neurosci 2012; 12: 251–318.
O'Tuathaigh CM, Kirby BP, Moran PM, Waddington JL . Mutant mouse models: genotype-phenotype relationships to negative symptoms in schizophrenia. Schizophr Bull 2010; 36: 271–288.
Porsolt RD, Bertin A, Jalfre M . Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977; 229: 327–336.
Cryan JF, Sweeney FF . The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol 2011; 164: 1129–1161.
Zohar J, Juven-Wetzler A, Sonnino R, Cwikel-Hamzany S, Balaban E, Cohen H . New insights into secondary prevention in post-traumatic stress disorder. Dialogues Clin Neurosci 2011; 13: 301–309.
Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L et al. Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology 2003; 28: 1031–1044.
File SE . The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods 1980; 2: 219–238.
Sanders SK, Shekhar A . Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry 1995; 37: 473–476.
Vilarim MM, Rocha Araujo DM, Nardi AE . Caffeine challenge test and panic disorder: a systematic literature review. Expert Rev Neurother 2011; 11: 1185–1195.
Palit G, Kumar R, Chowdhury SR, Gupta MB, Saxena RC, Srimal RC et al. A primate model of anxiety. Eur Neuropsychopharmacol 1998; 8: 195–201.
Kayir H, Uzbay IT . Nicotine antagonizes caffeine- but not pentylenetetrazole-induced anxiogenic effect in mice. Psychopharmacology (Berl) 2006; 184: 464–469.
Lovallo WR, Al'Absi M, Blick K, Whitsett TL, Wilson MF . Stress-like adrenocorticotropin responses to caffeine in young healthy men. Pharmacol Biochem Behav 1996; 55: 365–369.
Hale MW, Johnson PL, Westerman AM, Abrams JK, Shekhar A, Lowry CA . Multiple anxiogenic drugs recruit a parvalbumin-containing subpopulation of GABAergic interneurons in the basolateral amygdala. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 1285–1293.
Lu L, Mamiya T, Koseki T, Mouri A, Nabeshima T . Genetic animal models of schizophrenia related with the hypothesis of abnormal neurodevelopment. Biol Pharm Bull 2011; 34: 1358–1363.
Knight JC . Regulatory polymorphisms underlying complex disease traits. J Mol Med 2005; 83: 97–109.
Lesch KP . Gene-environment interaction and the genetics of depression. J Psychiatry Neurosci 2004; 29: 174–184.
Malan-Muller S, Hemmings SM, Seedat S . Big effects of small RNAs: a review of microRNAs in anxiety. Mol Neurobiol 2013; 47: 726–739.
McIlvain V, McCasland JS . GAP-43 heterozygous mice show delayed barrel patterning, differentiation of radial glia, and downregulation of GAP-43. Anat Rec A Discov Mol Cell Evol Biol 2006; 288A: 143–157.
Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 2002; 416: 396–400.
Lenze EJ, Wetherell JL . A lifespan view of anxiety disorders. Dialogues Clin Neurosci 2011; 13: 381–399.
Kas MJ, Krishnan V, Gould TD, Collier DA, Olivier B, Lesch KP et al. Advances in multidisciplinary and cross-species approaches to examine the neurobiology of psychiatric disorders. Eur Neuropsychopharmacol 2011; 21: 532–544.
Cryan JF, Holmes A . The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 2005; 4: 775–790.
Szekely M . The vagus nerve in thermoregulation and energy metabolism. Autonomic Neurosci 2000; 85: 26–38.
van Veen T, Goeman JJ, Monajemi R, Wardenaar KJ, Hartman CA, Snieder H et al. Different gene sets contribute to different symptom dimensions of depression and anxiety. Am J Med Genet B Neuropsychiatr Genet 2012; 159B: 519–528.
Luciano M, Huffman JE, Arias-Vásquez A, Vinkhuyzen AAE, Middeldorp CM, Giegling I et al. Genome-wide association uncovers shared genetic effects among personality traits and mood states. Am J Med Genet B Neuropsychiatric Genet 2012; 159B: 684–695.
Morris SE, Cuthbert BN . Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci 2012; 14: 29–37.
Kas MJ, Kahn RS, Collier DA, Waddington JL, Ekelund J, Porteous DJ et al. Translational neuroscience of Schizophrenia: seeking a meeting of minds between mouse and man. Sci Transl Med 2011; 3: 102mr103.
Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH . Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol Bull 2007; 133: 1–24.
Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A . Modulation of anxiety circuits by serotonergic systems. Stress 2005; 8: 233–246.
Duncan GE, Knapp DJ, Breese GR . Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res 1996; 713: 79–91.
Hale MW, Bouwknecht JA, Spiga F, Shekhar A, Lowry CA . Exposure to high- and low-light conditions in an open-field test of anxiety increases c-Fos expression in specific subdivisions of the rat basolateral amygdaloid complex. Brain Res Bull 2006; 71: 174–182.
Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Shekhar A, Lowry CA . Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience 2008; 155: 659–672.
Hafting T, Fyhn M, Molden S, Moser MB, Moser EI . Microstructure of a spatial map in the entorhinal cortex. Nature 2005; 436: 801–806.
Witter MP, Naber PA, van Haeften T, Machielsen WCM, SARB Rombouts, Barkhof F et al. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus 2000; 10: 398–410.
Bertoglio LJ, Joca SRL, Guimarães FS . Further evidence that anxiety and memory are regionally dissociated within the hippocampus. Behav Brain Res 2006; 175: 183–188.
Refojo D, Holsboer F . CRH signaling. Molecular specificity for drug targeting in the CNS. Ann N Y Acad Sci 2009; 1179: 106–119.
Nazari-Serenjeh F, Rezayof A . Cooperative interaction between the basolateral amygdala and ventral tegmental area modulates the consolidation of inhibitory avoidance memory. Prog Neuropsychopharmacol Biol Psychiatry 2013; 40: 54–61.
Carobrez AP, Bertoglio LJ . Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 2005; 29: 1193–1205.
Prediger RDS, Matheus FC, Schwarzbold ML, Lima MMS, Vital MABF . Anxiety in Parkinson’s disease: a critical review of experimental and clinical studies. Neuropharmacology 2012; 62: 115–124.
Skelly LR, Decety J . Passive and motivated perception of emotional faces: qualitative and quantitative changes in the face processing network. PLoS ONE 2012; 7: e40371.
Weathington JM, Strahan JA, Cooke BM . Social experience induces sex-specific fos expression in the amygdala of the juvenile rat. Horm Behav 2012; 62: 154–161.
Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 2011; 223: 403–410.
Markett S, Weber B, Voigt G, Montag C, Felten A, Elger C et al. Intrinsic connectivity networks and personality: the temperament dimension harm avoidance moderates functional connectivity in the resting brain. Neuroscience 2013; 240C: 98–105.
Franklin Tamara B, Saab Bechara J, Mansuy Isabelle M . Neural mechanisms of stress resilience and vulnerability. Neuron 2012; 75: 747–761.
Kofink D, Boks MP, Timmers HT, Kas MJ . Epigenetic dynamics in psychiatric disorders: environmental programming of neurodevelopmental processes. Neurosci Biobehav Rev 2013; 37: 831–845.
Kalkman HO . A review of the evidence for the canonical Wnt pathway in autism spectrum disorders. Mol Autism 2012; 3: 10.
Acknowledgements
This work was supported in part by a grant from the Israel Science Foundation (to BL). We thank Dr A Rigbi for his assistance in the statistical analysis of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Lotan, A., Lifschytz, T., Slonimsky, A. et al. Neural mechanisms underlying stress resilience in Ahi1 knockout mice: relevance to neuropsychiatric disorders. Mol Psychiatry 19, 243–252 (2014). https://doi.org/10.1038/mp.2013.123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.123
Keywords
This article is cited by
-
AHI1: linking depression and impaired antiviral immune response
Cell Research (2022)
-
Ahi1 regulates the nuclear translocation of glucocorticoid receptor to modulate stress response
Translational Psychiatry (2021)
-
Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex
Nature Communications (2020)
-
Amygdala hyper-connectivity in a mouse model of unpredictable early life stress
Translational Psychiatry (2018)
-
Social dominance predicts hippocampal glucocorticoid receptor recruitment and resilience to prenatal adversity
Scientific Reports (2018)