Abstract
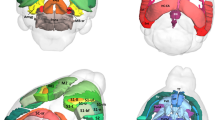
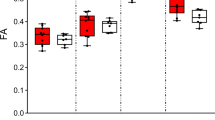
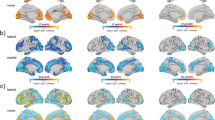
Recurrent deletions at the 22q11.2 locus have been established as a strong genetic risk factor for the development of schizophrenia and cognitive dysfunction. Individuals with 22q11.2 deletions have a range of well-defined volumetric abnormalities in a number of critical brain structures. A mouse model of the 22q11.2 deletion (Df(16)A+/−) has previously been utilized to characterize disease-associated abnormalities on synaptic, cellular, neurocircuitry, and behavioral levels. We performed a high-resolution MRI analysis of mutant mice compared with wild-type littermates. Our analysis revealed a striking similarity in the specific volumetric changes of Df(16)A+/− mice compared with human 22q11.2 deletion carriers, including in cortico-cerebellar, cortico-striatal and cortico-limbic circuits. In addition, higher resolution magnetic resonance imaging compared with neuroimaging in human subjects allowed the detection of previously unknown subtle local differences. The cerebellar findings in Df(16)A+/− mice are particularly instructive as they are localized to specific areas within both the deep cerebellar nuclei and the cerebellar cortex. Our study indicates that the Df(16)A+/−mouse model recapitulates most of the hallmark neuroanatomical changes observed in 22q11.2 deletion carriers. Our findings will help guide the design and interpretation of additional complementary studies and thereby advance our understanding of the abnormal brain development underlying the emergence of 22q11.2 deletion-associated psychiatric and cognitive symptoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Karayiorgou M, Simon TJ, Gogos JA . 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci 2010; 11: 402–416.
Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry 2011; 168: 302–316.
Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA 1995; 92: 7612–7616.
Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M . Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet 2008; 40: 880–885.
Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T et al. Strong association of de novo copy number mutations with autism. Science 2007; 316: 445–449.
Malhotra D, McCarthy S, Michaelson JJ, Vacic V, Burdick KE, Yoon S et al. High frequencies of de novo CNVs in bipolar disorder and schizophrenia. Neuron 2011; 72: 951–963.
Stark KL, Xu B, Bagchi A, Lai W-S, Liu H, Hsu R et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet 2008; 40: 751–760.
Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, Macdermott AB et al. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci 2008; 11: 1302–1310.
Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA . Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 2010; 464: 763–767.
Fénelon K, Mukai J, Xu B, Hsu P-K, Drew LJ, Karayiorgou M et al. Deficiency of Dgcr8, a gene disrupted by the 22q11.2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proc Natl Acad Sci USA 2011; 108: 4447–4452.
Drew LJ, Stark KL, Fénelon K, Karayiorgou M, Macdermott AB, Gogos JA . Evidence for altered hippocampal function in a mouse model of the human 22q11.2 microdeletion. Mol Cell Neurosci 2011; 47: 293–305.
Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y et al. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat Genet 2012; 44: 1365–1369.
Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B et al. The 22q11.2 microdeletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int J Dev Neurosci 2011; 29: 259–281.
Arguello PA, Gogos JA . Modeling madness in mice: one piece at a time. Neuron 2006; 52: 179–196.
Zhang J, Peng Q, Li Q, Jahanshad N, Hou Z, Jiang M et al. Longitudinal characterization of brain atrophy of a Huntington's disease mouse model by automated morphological analyses of magnetic resonance images. Neuroimage 2010; 49: 2340–2351.
Cramer PE, Cirrito JR, Wesson DW, Lee CYD, Karlo JC, Zinn AE et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 2012; 335: 1503–1506.
Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron 2005; 45: 489–496.
Lerch JP, Yiu AP, Martinez-Canabal A, Pekar T, Bohbot VD, Frankland PW et al. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage 2011; 54: 2086–2095.
Nieman BJ, Wong MD, Henkelman RM . Genes into geometry: imaging for mouse development in 3D. Curr Opini Genet Dev 2011; 21: 638–646.
Nieman BJ, Lerch JP, Bock NA, Chen XJ, Sled JG, Henkelman RM . Mouse behavioral mutants have neuroimaging abnormalities. Hum Brain Mapp 2007; 28: 567–575.
Ellegood J, Pacey LK, Hampson DR, Lerch JP, Henkelman RM . Anatomical phenotyping in a mouse model of fragile X syndrome with magnetic resonance imaging. Neuroimage 2010; 53: 1023–1029.
Horev G, Ellegood J, Lerch JP, Son Y-EE, Muthuswamy L, Vogel H et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc Natl Acad Sci USA 2011; 108: 17076–17081.
Ellegood J, Lerch JP, Henkelman RM . Brain abnormalities in a Neuroligin3 R451C knocking mouse model associated with autism. Autism Res 2011; 4: 368–376.
Tan GM, Arnone D, McIntosh AM, Ebmeier KP . Meta-analysis of magnetic resonance imaging studies in chromosome 22q11.2 deletion syndrome (velocardiofacial syndrome). Schizophrenia Res 2009; 115: 173–181.
Johnson GA, Cofer GP, Fubara B, Gewalt SL, Hedlund LW, Maronpot RR . Magnetic resonance histology for morphologic phenotyping. J Magn Reson Imaging 2002; 16: 423–429.
Lerch JP, Sled JG, Henkelman RM . MRI phenotyping of genetically altered mice. Methods Mol Biol 2011; 711: 349–361.
Dazai J, Spring S, Cahill LS, Henkelman RM . Multiple-mouse neuroanatomical magnetic resonance imaging. J Vis Exp 2011; 48: 2497.
Thomas DL, De Vita E, Roberts S, Turner R, Yousry TA, Ordidge RJ . High-resolution fast spin echo imaging of the human brain at 4.7T: implementation and sequence characteristics. Magn Reson Med 2004; 51: 1254–1264.
Collins DL, Neelin P, Peters TM, Evans AC . Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 1994; 18: 192–205.
Avants BB, Epstein CL, Grossman M, Gee JC . Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008; 12: 26–41.
Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC . A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011; 54: 2033–2044.
Nieman BJ, Flenniken AM, Adamson SL, Henkelman RM, Sled JG . Anatomical phenotyping in the brain and skull of a mutant mouse by magnetic resonance imaging and computed tomography. Physiol Genomics 2006; 24: 154–162.
Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM . High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 2008; 42: 60–69.
Genovese CR, Lazar NA, Nichols T . Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002; 15: 870–878.
Smith SM, Nichols TE . Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44: 83–98.
Deboer T, Wu Z, Lee A, Simon TJ . Hippocampal volume reduction in children with chromosome 22q11.2 deletion syndrome is associated with cognitive impairment. Behav Brain Funct 2007; 3: 54.
Gothelf D, Michaelovsky E, Frisch A, Zohar AH, Presburger G, Burg M et al. Association of the low-activity COMT 158Met allele with ADHD and OCD in subjects with velocardiofacial syndrome. Int J Neuropsychopharmacol 2007; 10: 301–308.
Kates WR, Burnette CP, Bessette BA, Folley BS, Strunge L, Jabs EW et al. Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2). J Child Neurol 2004; 19: 337–342.
Eliez S, Barnea-Goraly N, Schmitt JE, Liu Y, Reiss AL . Increased basal ganglia volumes in velo-cardio-facial syndrome (deletion 22q11.2). BPS 2002; 52: 68–70.
Kates WR, Bansal R, Fremont W, Antshel KM, Hao X, Higgins AM et al. Mapping cortical morphology in youth with velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry 2011; 50: e272.
Chow EWC, Zipursky RB, Mikulis DJ, Bassett AS . Structural brain abnormalities in patients with schizophrenia and 22q11 deletion syndrome. BPS 2002; 51: 208–215.
Eliez S, Schmitt JE, White CD, Reiss AL . Children and adolescents with velocardiofacial syndrome: a volumetric MRI study. Am J Psychiatry 2000; 157: 409–415.
van Amelsvoort T, Daly E, Robertson D, Suckling J, Ng V, Critchley H et al. Structural brain abnormalities associated with deletion at chromosome 22q11: quantitative neuroimaging study of adults with velo-cardio-facial syndrome. Br J Psychiatry 2001; 178: 412–419.
Chow EW, Mikulis DJ, Zipursky RB, Scutt LE, Weksberg R, Bassett AS . Qualitative MRI findings in adults with 22q11 deletion syndrome and schizophrenia. BPS 1999; 46: 1436–1442.
Sugama S, Bingham PM, Wang PP, Moss EM, Kobayashi H, Eto Y . Morphometry of the head of the caudate nucleus in patients with velocardiofacial syndrome (del 22q11.2). Acta Paediatr 2000; 89: 546–549.
Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE . MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry 2000; 157: 281–283.
Karayiorgou M, Sobin C, Blundell ML, Galke BL, Malinova L, Goldberg P et al. Family-based association studies support a sexually dimorphic effect of COMT and MAOA on genetic susceptibility to obsessive-compulsive disorder. BPS 1999; 45: 1178–1189.
Garrett A, Penniman L, Epstein JN, Casey BJ, Hinshaw SP, Glover G et al. Neuroanatomical abnormalities in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2008; 47: 1321–1328.
Sobin C, Kiley-Brabeck K, Daniels S, Khuri J, Taylor L, Blundell M et al. Neuropsychological characteristics of children with the 22q11 Deletion Syndrome: a descriptive analysis. Child Neuropsychol 2005; 11: 39–53.
Shenton ME, Dickey CC, Frumin M, McCarley RW . A review of MRI findings in schizophrenia. Schizophrenia Res 2001; 49: 1–52.
Bish JP, Pendyal A, Ding L, Ferrante H, Nguyen V, McDonald-McGinn D et al. Specific cerebellar reductions in children with chromosome 22q11.2 deletion syndrome. Neurosci Lett 2006; 399: 245–248.
Rasser PE, Schall U, Peck G, Cohen M, Johnston P, Khoo K et al. Cerebellar grey matter deficits in first-episode schizophrenia mapped using cortical pattern matching. Neuroimage 2010; 53: 1175–1180.
Ilg UJ, Thier P . The neural basis of smooth pursuit eye movements in the rhesus monkey brain. Brain Cogn 2008; 68: 229–240.
Vorstman JAS, Turetsky BI, Sijmens-Morcus MEJ . de Sain MG, Dorland B, Sprong M et al. Proline affects brain function in 22q11DS children with the low activity COMT 158 allele. Neuropsychopharmacology 2009; 34: 739–746.
Park BL, Shin HD, Cheong HS, Park CS, Sohn J-W, Kim B-J et al. Association analysis of COMT polymorphisms with schizophrenia and smooth pursuit eye movement abnormality. J Hum Genet 2009; 54: 709–712.
Shin HD, Park BL, Bae JS, Park TJ, Chun JY, Park CS et al. Association of ZDHHC8 polymorphisms with smooth pursuit eye movement abnormality. Am J Med Genet B Neuropsychiatr Genet 2010; 153B: 1167–1172.
Mukai J, Liu H, Burt RA, Swor DE, Lai W-S, Karayiorgou M et al. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat Genet 2004; 36: 725–731.
Levy DL, Sereno AB, Gooding DC, O'Driscoll GA . Eye tracking dysfunction in schizophrenia: characterization and pathophysiology. Curr Top Behav Neurosci 2010; 4: 311–347.
Ramnani N . The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci 2006; 7: 511–522.
Stoodley CJ, Schmahmann JD . Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 2009; 44: 489–501.
Stoodley CJ, Valera EM, Schmahmann JD . Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 2012; 59: 1560–1570.
Arenz A, Bracey EF, Margrie TW . Sensory representations in cerebellar granule cells. Curr Opin Neurobiol 2009; 19: 445–451.
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010; 468: 270–276.
Fung WLA, McEvilly R, Fong J . Silversides C, Chow E, Bassett A. Elevated prevalence of generalized anxiety disorder in adults with 22q11.2 deletion syndrome. Am J Psychiatry 2010; 167: 998.
Andersson F, Glaser B, Spiridon M, Debbané M, Vuilleumier P, Eliez S . Impaired activation of face processing networks revealed by functional magnetic resonance imaging in 22q11.2 deletion syndrome. Biol Psychiatry 2008; 63: 49–57.
Namiki C, Hirao K, Yamada M, Hanakawa T, Fukuyama H, Hayashi T et al. Impaired facial emotion recognition and reduced amygdalar volume in schizophrenia. Psychiatry Res 2007; 156: 23–32.
Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI et al. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry 2002; 159: 1992–1999.
Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR . Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry 2012; 69: 893–903.
Harper KM, Hiramoto T, Tanigaki K, Kang G, Suzuki G, Trimble W et al. Alterations of social interaction through genetic and environmental manipulation of the 22q11.2 gene Sept5 in the mouse brain. Hum Mol Genet 2012; 21: 3489–3499.
Shashi V, Kwapil TR, Kaczorowski J, Berry MN, Santos CS, Howard TD et al. Evidence of gray matter reduction and dysfunction in chromosome 22q11.2 deletion syndrome. Psychiatry Res 2010; 181: 1–8.
Bearden CE, van Erp TGM, Dutton RA, Tran H, Zimmermann L, Sun D et al. Mapping cortical thickness in children with 22q11.2 deletions. Cereb Cortex 2007; 17: 1889–1898.
Eliez S, Schmitt JE, White CD, Wellis VG, Reiss AL . A quantitative MRI study of posterior fossa development in velocardiofacial syndrome. BPS 2001; 49: 540–546.
van Amelsvoort T, Daly E, Henry J, Robertson D, Ng V, Owen M et al. Brain anatomy in adults with velocardiofacial syndrome with and without schizophrenia: preliminary results of a structural magnetic resonance imaging study. Arch Gen Psychiatry 2004; 61: 1085–1096.
Rimol LM, Hartberg CB, Nesvåg R, Fennema-Notestine C, Hagler DJ, Pung CJ et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biological Psychiatry 2010; 68: 41–50.
Watson DR, Anderson JME, Bai F, Barrett SL, McGinnity TM, Mulholland CC et al. A voxel based morphometry study investigating brain structural changes in first episode psychosis. Behav Brain Res 2012; 227: 91–99.
Greenstein D, Lenroot R, Clausen L, Chavez A, Vaituzis AC, Tran L et al. Cerebellar development in childhood onset schizophrenia and non-psychotic siblings. Psychiatry Res 2011; 193: 131–137.
Bergé D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O . Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand 2011; 123: 431–439.
Borgwardt SJ, Picchioni MM, Ettinger U, Toulopoulou T, Murray R, McGuire PK . Regional gray matter volume in monozygotic twins concordant and discordant for schizophrenia. Biol Psychiatry 2010; 67: 956–964.
Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Zoltick B et al. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry 2008; 63: 475–483.
Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG . Structural analysis of the basal ganglia in schizophrenia. Schizophrenia Res 2007; 89: 59–71.
Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151: 1430–1436.
Sieberer M, Haltenhof H, Haubitz B, Pabst B, Miller K, Garlipp P . Basal ganglia calcification and psychosis in 22q11.2 deletion syndrome. Eur Psychiatry 2005; 20: 567–569.
Cao Z, Yu R, Dun K, Burke J, Caplin N, Greenaway T . 22q11.2 deletion presenting with severe hypocalcaemia, seizure and basal ganglia calcification in an adult man. Intern Med J 2011; 41: 63–66.
Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D et al. Caudate, putamen, and globus pallidus volume in schizophrenia: a quantitative MRI study. Psychiatry Res 1995; 61: 209–229.
Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM . Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry 2009; 195: 194–201.
Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J . Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neuroimage 2005; 25: 169–180.
Galderisi S, Quarantelli M, Volpe U, Mucci A, Cassano GB, Invernizzi G et al. Patterns of structural MRI abnormalities in deficit and nondeficit schizophrenia. Schizophr Bull 2008; 34: 393–401.
Styner M, Lieberman JA, McClure RK, Weinberger DR, Jones DW, Gerig G . Morphometric analysis of lateral ventricles in schizophrenia and healthy controls regarding genetic and disease-specific factors. Proc Natl Acad Sci USA 2005; 102: 4872–4877.
Exner C, Boucsein K, Degner D, Irle E, Weniger G . Impaired emotional learning and reduced amygdala size in schizophrenia: a 3-month follow-up. Schizophrenia Res 2004; 71: 493–503.
Yoshida T, McCarley RW, Nakamura M, Lee K, Koo M-S, Bouix S et al. A prospective longitudinal volumetric MRI study of superior temporal gyrus gray matter and amygdala-hippocampal complex in chronic schizophrenia. Schizophrenia Res 2009; 113: 84–94.
James AC, James S, Smith DM, Javaloyes A . Cerebellar, prefrontal cortex, and thalamic volumes over two time points in adolescent-onset schizophrenia. Am J Psychiatry 2004; 161: 1023–1029.
Yoshihara Y, Sugihara G, Matsumoto H, Suckling J, Nishimura K, Toyoda T et al. Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Ann Gen Psychiatry 2008; 7: 25.
Cascella NG, Fieldstone SC, Rao VA, Pearlson GD, Sawa A, Schretlen DJ . Gray-matter abnormalities in deficit schizophrenia. Schizophrenia Res 2010; 120: 63–70.
Mané A, Falcon C, Mateos JJ, Fernandez-Egea E, Horga G, Lomeña F et al. Progressive gray matter changes in first episode schizophrenia: a 4-year longitudinal magnetic resonance study using VBM. Schizophrenia Res 2009; 114: 136–143.
Neckelmann G, Specht K, Lund A, Ersland L, Smievoll AI, Neckelmann D et al. Mr morphometry analysis of grey matter volume reduction in schizophrenia: association with hallucinations. Int J Neurosci 2006; 116: 9–23.
Douaud G, Mackay C, Andersson J, James S, Quested D, Ray MK et al. Schizophrenia delays and alters maturation of the brain in adolescence. Brain 2009; 132: 2437–2448.
Rotarska-Jagiela A, Oertel-Knoechel V, DeMartino F, van de Ven V, Formisano E, Roebroeck A et al. Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res 2009; 174: 9–16.
Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE . Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry 2000; 157: 828–830.
Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC . An MRI study of midbrain morphology in patients with schizophrenia: relationship to psychosis, neuroleptics, and cerebellar neural circuitry. BPS 2001; 49: 13–19.
Koolschijn PC, van Haren NE, Hulshoff Pol HE, Kahn RS . Hypothalamus volume in twin pairs discordant for schizophrenia. Eur Neurol 2008; 18: 312–315.
Acknowledgements
We thank Louise van der Weerd for helpful discussions during the initial phase of this project and Christine LaLiberté, Kim Stark and Matthijs van Eede for their assistance with aspects of the MRI scanning and analysis. We would also like to acknowledge the Ontario Mental Health Foundation (OHMF) for salary support (JE). This work was supported by grants from the US National Institute of Mental Health, Grants MH67068 (to MK and JAG) and MH077235 (to JAG) and the Canadian Institute for Health Research (CIHR) and the Ontario Brain Institute (OBI).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Ellegood, J., Markx, S., Lerch, J. et al. Neuroanatomical phenotypes in a mouse model of the 22q11.2 microdeletion. Mol Psychiatry 19, 99–107 (2014). https://doi.org/10.1038/mp.2013.112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.112
Keywords
This article is cited by
-
Mitochondrial proteins encoded by the 22q11.2 neurodevelopmental locus regulate neural stem and progenitor cell proliferation
Molecular Psychiatry (2023)
-
Neuroanatomy and behavior in mice with a haploinsufficiency of AT-rich interactive domain 1B (ARID1B) throughout development
Molecular Autism (2021)
-
Schizophrenia-related microdeletion causes defective ciliary motility and brain ventricle enlargement via microRNA-dependent mechanisms in mice
Nature Communications (2020)
-
Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: Convergence with idiopathic psychosis and effects of deletion size
Molecular Psychiatry (2020)
-
The autism- and schizophrenia-associated protein CYFIP1 regulates bilateral brain connectivity and behaviour
Nature Communications (2019)