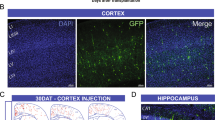
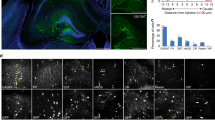
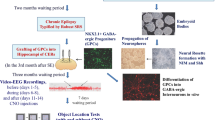
Abstract
Schizophrenia patients exhibit increased hippocampal activity that is correlated with positive symptoms. Although the cause of this hippocampal hyperactivity has not been demonstrated, it likely involves a decrease in GABAergic signaling. Thus, we posit that restoring GABAergic function may provide a novel therapeutic approach for the treatment of schizophrenia. It has been demonstrated that transplanted GABAergic precursor cells from the medial ganglionic eminence (MGE) can migrate and differentiate into mature interneurons. Here, we demonstrate that ventral hippocampal MGE transplants can restore hippocampal function and normalize downstream dopamine neuron activity in a rodent model of schizophrenia. Furthermore, MGE transplants also reverse the hyper-responsive locomotor response to amphetamine. Taken together, these data demonstrate that restoring interneuron function reverses neurophysiological and behavioral deficits in a rodent model of schizophrenia and moreover, demonstrate the feasibility of a neuronal transplant procedure as a potential novel therapeutic approach for the treatment of schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Laruelle M, Abi-Dargham A . Dopamine as the wind of psychotic fire: new evidence from brain imaging studies. J Psychopharmacol 1999; 13: 358–371.
Abi-Dargham A . Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol 2004; 7 (Suppl 1): S1–S5.
Lodge DJ, Grace AA . Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 2007; 27: 11424–11430.
Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R . Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology 2006; 31: 221–230.
Medoff DR, Holcomb HH, Lahti AC, Tamminga CA . Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus 2001; 11: 543–550.
Harrison PJ . The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004; 174: 151–162.
Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 2009; 66: 938–946.
Perez SM, Shah A, Asher A, Lodge DJ . Hippocampal deep brain stimulation reverses physiological and behavioral deficits in a rodent model of schizophrenia. Int J Neuropsychopharmacol 2012; 16: 1331–1339.
Shah A, Lodge DJ . A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry 2013; 3: e215.
Lewis DA, Hashimoto T, Volk DW . Cortical inhibitory neurons and schizophrenia. Nat Rev Neuroscience 2005; 6: 312–324.
Lodge DJ, Behrens MM, Grace AA . A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci 2009; 29: 2344–2354.
Ducharme G, Lowe GC, Goutagny R, Williams S . Early alterations in hippocampal circuitry and theta rhythm generation in a mouse model of prenatal infection: implications for schizophrenia. PLoS One 2012; 7: e29754.
Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA . A novel α5GABAAR-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology 2011; 36: 1903–1911.
Lodge DJ, Grace AA . Gestational methylazoxymethanol acetate administration: A developmental disruption model of schizophrenia. Behav Brain Res 2009; 7 204: 306–312.
Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci USA 2009; 106: 15472–15477.
Martinez-Cerdeno V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned Rats. Cell Stem Cell 2010; 6: 238–250.
Tanaka DH, Toriumi K, Kubo K, Nabeshima T, Nakajima K . GABAergic precursor transplantation into the prefrontal cortex prevents phencyclidine-induced cognitive deficits. J Neurosci 2011; 31: 14116–14125.
Calcagnotto ME, Zipancic I, Piquer-Gil M, Mello LE, Alvarez-Dolado M . Grafting of GABAergic precursors rescues deficits in hippocampal inhibition. Epilepsia 2010; 51 (SUPPL 3): 66–70.
Alvarez-Dolado M, Calcagnotto ME, Karkar KM, Southwell DG, Jones-Davis DM, Estrada RC et al. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci 2006; 26: 7380–7389.
Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A . Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci 1999; 2: 461–466.
Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA . A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry 2006; 60: 253–264.
Ranck JB Jr . Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol 1973; 41: 461–531.
Van Der Meer MAA, Redish AD . Theta phase precession in rat ventral striatum links place and reward information. J Neurosci 2011; 31: 2843–2854.
Grace AA, Bunney BS . Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience 1983; 10: 301–315.
Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1998; 1: 318–323.
Gonzalez-Burgos G, Hashimoto T, Lewis DA . Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep 2010; 12: 335–344.
Perry TL, Kish SJ, Buchanan J, Hansen S . Gamma-aminobutyric-acid deficiency in brain of schizophrenic patients. Lancet 1979; 1: 237–239.
Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, Seckinger R, Goetz R et al. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophrenia Res 2009; 114: 110–118.
Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 2003; 23: 6315–6326.
Curley AA, Eggan SM, Lazarus MS, Huang ZJ, Volk DW, Lewis DA . Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: Implications for schizophrenia. Neurobiol Dis 2013; 50: 179–186.
Lodge DJ, Grace AA . Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 2011; 32: 507–513.
Grace AA, Floresco SB, Goto Y, Lodge DJ . Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 2007; 30: 220–227.
Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, De Bartolomeis A et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 1997; 94: 2569–2574.
Swerdlow NR, Halim N, Hanlon FM, Platten A, Auerbach PP . Lesion size and amphetamine hyperlocomotion after neonatal ventral hippocampal lesions: more is less. Brain Res Bull 2001; 55: 71–77.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates. Academic Press: Sydney, 1986.
Acknowledgements
This work was supported by a mental health research grant from the Hogg Foundation and an R01 (MH090067) and F31 (MH098564) from the NIH. Representative images were generated in the Core Optical Imaging Facility which is supported by UTHSCSA, NIH-NCI P30 CA54174 (CTRC at UTHSCSA) and NIH-NIA P01AG19316.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Lodge reports receiving consulting fees from Dey Pharmaceuticals, whereas Perez declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Perez, S., Lodge, D. Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Mol Psychiatry 18, 1193–1198 (2013). https://doi.org/10.1038/mp.2013.111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.111
Keywords
This article is cited by
-
Discrete hippocampal projections are differentially regulated by parvalbumin and somatostatin interneurons
Nature Communications (2023)
-
Potential Plausible Role of Stem Cell for Treating Depressive Disorder: a Retrospective Review
Molecular Neurobiology (2023)
-
Hippocampal circuit dysfunction in psychosis
Translational Psychiatry (2022)
-
Adult stress exposure blunts dopamine system hyperresponsivity in a neurodevelopmental rodent model of schizophrenia
Schizophrenia (2022)
-
Dopaminergic dysfunction and excitatory/inhibitory imbalance in treatment-resistant schizophrenia and novel neuromodulatory treatment
Molecular Psychiatry (2022)