Abstract
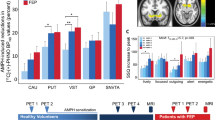
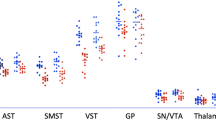
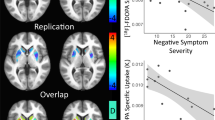
Dopamine (DA) has a role in the pathophysiology of schizophrenia and addiction. Imaging studies have indicated that striatal DA release is increased in schizophrenia, predominantly in the precommissural caudate (preDCA), and blunted in addiction, mostly in the ventral striatum (VST). Therefore, we aimed to measure striatal DA release in patients with comorbid schizophrenia and substance dependence. We used [11C]raclopride positron emission tomography and an amphetamine challenge to measure baseline DA D2-receptor availability (BPND) and its percent change post-amphetamine (ΔBPND, to index amphetamine-induced DA release) in striatal subregions in 11 unmedicated, drug-free patients with both schizophrenia and substance dependence, and 15 healthy controls. There were no significant group differences in baseline BPND. Linear mixed modeling using ΔBPND as the dependent variable and striatal region of interest as a repeated measure indicated a significant main effect of diagnosis, F(1, 24)=8.38, P=0.008, with significantly smaller ΔBPND in patients in all striatal subregions (all P⩽0.04) except VST. Among patients, change in positive symptoms after amphetamine was significantly associated with ΔBPND in the preDCA (rs=0.69, P=0.03) and VST (rs=0.64, P=0.05). In conclusion, patients with comorbid schizophrenia and substance dependence showed significant blunting of striatal DA release, in contrast to what has been found in schizophrenia without substance dependence. Despite this blunting, DA release was associated with the transient amphetamine-induced positive-symptom change, as observed in schizophrenia. This is the first description of a group of patients with schizophrenia who display low presynaptic DA release, yet show a psychotic reaction to increases in D2 stimulation, suggesting abnormal postsynaptic D2 function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA 1996; 93: 9235–9240.
Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 1998; 155: 761–767.
Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 1997; 94: 2569–2574.
Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R . Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 1999; 46: 56–72.
Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 2007; 164: 622–629.
Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 2005; 58: 779–786.
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 1997; 386: 830–833.
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001; 21: 1034–1057.
Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 2010; 67: 231–239.
Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990; 264: 2511–2518.
Swartz MS, Wagner HR, Swanson JW, Stroup TS, McEvoy JP, McGee M et al. Substance use and psychosocial functioning in schizophrenia among new enrollees in the NIMH CATIE study. Psychiatr Serv 2006; 57: 1110–1116.
Swartz MS, Wagner HR, Swanson JW, Stroup TS, McEvoy JP, Canive JM et al. Substance use in persons with schizophrenia: baseline prevalence and correlates from the NIMH CATIE study. J Nerv Ment Dis 2006; 194: 164–172.
Swendsen J, Ben-Zeev D, Granholm E . Real-time electronic ambulatory monitoring of substance use and symptom expression in schizophrenia. Am J Psychiatry 2011; 168: 202–209.
Cantwell R, Brewin J, Glazebrook C, Dalkin T, Fox R, Medley I et al. Prevalence of substance misuse in first-episode psychosis. Br J Psychiatry 1999; 174: 150–153.
Van Mastrigt S, Addington J, Addington D . Substance misuse at presentation to an early psychosis program. Soc Psychiatry Psychiatr Epidemiol 2004; 39: 69–72.
Barnett JH, Werners U, Secher SM, Hill KE, Brazil R, Masson K et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry 2007; 190: 515–520.
Lambert M, Conus P, Lubman DI, Wade D, Yuen H, Moritz S et al. The impact of substance use disorders on clinical outcome in 643 patients with first-episode psychosis. Acta Psychiatr Scand 2005; 112: 141–148.
Verdoux H, Liraud F, Gonzales B, Assens F, Abalan F, van Os J . Suicidality and substance misuse in first-admitted subjects with psychotic disorder. Acta Psychiatr Scand 1999; 100: 389–395.
Schiffer B, Muller BW, Scherbaum N, Forsting M, Wiltfang J, Leygraf N et al. Impulsivity-related brain volume deficits in schizophrenia-addiction comorbidity. Brain 2010; 133: 3093–3103.
Ebdrup BH, Glenthoj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB et al. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci 2010; 35: 95–104.
Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV . Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch Gen Psychiatry 2003; 60: 245–252.
Wobrock T, Sittinger H, Behrendt B, D’Amelio R, Falkai P . Comorbid substance abuse and brain morphology in recent-onset psychosis. Eur Arch Psychiatry Clin Neurosci 2009; 259: 28–36.
Hasin D, Samet S, Nunes E, Meydan J, Matseoane K, Waxman R . Diagnosis of comorbid psychiatric disorders in substance users assessed with the psychiatric research interview for substance and mental disorders for DSM-IV. Am J Psychiatry 2006; 163: 689–696.
Nurnberger Jr JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH genetics initiative. Arch Gen Psychiatry 1994; 51: 849–859.
First MB, Spitzer RL, Gibbon M, Williams JBW . Structured Clinical Interview for DSM-IV Axis I Disorders - Non-Patient Edition. New York Biometrics Research: New York State Psychiatric Institute, New York, NY, USA, 1996.
Hollingshead AB . Four Factor Index of Social Status, Working paper Connecticut, 1975 (Unpublished Working Paper, Department of Sociology, Yale University, New Haven, CT, USA).
Kay SR, Fiszbein A, Opler LA . The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13: 261–276.
Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE . Measurement of dopamine release with continuous infusion of [11C]raclopride: Optimization and signal-to-noise considerations. J Nucl Med 2000; 41: 522–530.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003; 23: 285–300.
van Kammen DP, Murphy DL . Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia 1975; 44: 215–224.
Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS et al. SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med 1995; 36: 1182–1190.
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 2000; 97: 8104–8109.
Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R et al. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry 2009; 166: 1170–1177.
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 2007; 27: 12700–12706.
Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S et al. Dopamine release in chronic cannabis users: A [(11)C]raclopride positron emission tomography study. Biol Psychiatry 2012; 71: 677–683.
Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H et al. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry 2008; 165: 507–514.
Busto UE, Redden L, Mayberg H, Kapur S, Houle S, Zawertailo LA . Dopaminergic activity in depressed smokers: a positron emission tomography study. Synapse 2009; 63: 681–689.
Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F . Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 2007; 64: 1575–1579.
Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F . Imaging dopamine's role in drug abuse and addiction. Neuropharmacology 2009; 56 (Suppl 1): 3–8.
Michaelides M, Thanos PK, Kim R, Cho J, Ananth M, Wang GJ et al. PET imaging predicts future body weight and cocaine preference. Neuroimage 2012; 59: 1508–1513.
Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment: Meta-analysis of imaging studies. Arch Gen Psychiatry 2012: doi:10.1001/archgenpsychiatry.2012.1169.
Schubert KO, Focking M, Prehn JH, Cotter DR . Hypothesis review: are clathrin-mediated endocytosis and clathrin-dependent membrane and protein trafficking core pathophysiological processes in schizophrenia and bipolar disorder? Mol Psychiatry 2011; 0: 1–13.
Acknowledgements
We thank the research participants of this study and express gratitude for the expert assistance of Beatriz Alvarez, Rawad Ayoub, Jennifer Bae, Felipe Castillo, John Castrillon, Ray Goetz, Elizabeth Hackett, Deborah Hasin, Olga Kambalov, Olga Medina, Elizabeth Raggi and Sharon Samet. This work was supported by a grant from the National Institute on Drug Abuse [R21 DA023039-01] to Anissa Abi-Dargham.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Thompson, J., Urban, N., Slifstein, M. et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol Psychiatry 18, 909–915 (2013). https://doi.org/10.1038/mp.2012.109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2012.109
Keywords
This article is cited by
-
Childhood traumatic events and the dopaminergic theory of psychosis: A mini-review of studies investigating gene – environment interactions
Current Psychology (2023)
-
Clinical correlation but no elevation of striatal dopamine synthesis capacity in two independent cohorts of medication-free individuals with schizophrenia
Molecular Psychiatry (2022)
-
Beyond antipsychotics: a twenty-first century update for preclinical development of schizophrenia therapeutics
Translational Psychiatry (2022)
-
Glutamatergic and dopaminergic function and the relationship to outcome in people at clinical high risk of psychosis: a multi-modal PET-magnetic resonance brain imaging study
Neuropsychopharmacology (2020)
-
Amphetamine-induced striatal dopamine release in schizotypal personality disorder
Psychopharmacology (2020)