Abstract
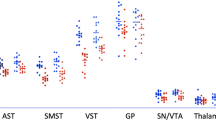
Studies in methamphetamine (METH) abusers showed that the decreases in brain dopamine (DA) function might recover with protracted detoxification. However, the extent to which striatal DA function in METH predicts recovery has not been evaluated. Here we assessed whether striatal DA activity in METH abusers is associated with clinical outcomes. Brain DA D2 receptor (D2R) availability was measured with positron emission tomography and [11C]raclopride in 16 METH abusers, both after placebo and after challenge with 60 mg oral methylphenidate (MPH) (to measure DA release) to assess whether it predicted clinical outcomes. For this purpose, METH abusers were tested within 6 months of last METH use and then followed up for 9 months of abstinence. In parallel, 15 healthy controls were tested. METH abusers had lower D2R availability in caudate than in controls. Both METH abusers and controls showed decreased striatal D2R availability after MPH and these decreases were smaller in METH than in controls in left putamen. The six METH abusers who relapsed during the follow-up period had lower D2R availability in dorsal striatum than in controls, and had no D2R changes after MPH challenge. The 10 METH abusers who completed detoxification did not differ from controls neither in striatal D2R availability nor in MPH-induced striatal DA changes. These results provide preliminary evidence that low striatal DA function in METH abusers is associated with a greater likelihood of relapse during treatment. Detection of the extent of DA dysfunction may be helpful in predicting therapeutic outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Seiden LS, Sabol KE . Methamphetamine and methylenedioxymethamphetamine neurotoxicity: possible mechanisms of cell destruction. NIDA Res Monogr 1996; 163: 251–276.
Woolverton WL, Ricaurte GA, Forno LS, Seiden LS . Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res 1989; 486: 73–78.
Krasnova IN, Cadet JL . Methamphetamine toxicity and messengers of death. Brain Res Rev 2009; 60: 379–407.
Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG et al. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci 2006; 31: 301–313.
Walsh SL, Wagner GC . Motor impairments after methamphetamine-induced neurotoxicity in the rat. J Pharmacol Exp Ther 1992; 263: 617–626.
Itoh J, Nabeshima T, Kameyama T . Utility of an elevated plus-maze for dissociation of amnesic and behavioral effects of drugs in mice. Eur J Pharmacol 1991; 194: 71–76.
Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza II R, Traynor JR, Woods JH . A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience 2008; 151: 533–543.
Thomas DM, Kuhn DM . Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J Neurochem 2005; 92: 790–797.
McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse 2008; 62: 91–100.
Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 2009; 29: 14734–14740.
Cadet JL, Jayanthi S, Deng X . Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Review. Neurotox Res 2005; 8: 199–206.
Seiden LS, Fischman MW, Schuster CR . Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend 1976; 1: 215–219.
Cass WA, Manning MW . Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J Neurosci 1999; 19: 7653–7660.
Friedman SD, Castaneda E, Hodge GK . Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav 1998; 61: 35–44.
Harvey DC, Lacan G, Tanious SP, Melega WP . Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res 2000; 871: 259–270.
Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 2001; 21: 9414–9418.
Narendran R, Martinez D . Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse 2008; 62: 851–869.
Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse 2002; 46: 79–82.
Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2007; 64: 932–940.
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 2007; 27: 12700–12706.
Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F . Quantification of behavior sackler colloquium: addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci USA; published online 14 March 2011; e-pub ahead of print.
Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001; 21: RC121.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990; 10: 740–747.
Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007; 28: 1194–1205.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000; 10: 120–131.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–289.
Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 2001; 158: 2015–2021.
Xu W, Zhu JP, Angulo JA . Induction of striatal pre- and postsynaptic damage by methamphetamine requires the dopamine receptors. Synapse 2005; 58: 110–121.
Tong J, Ross BM, Schmunk GA, Peretti FJ, Kalasinsky KS, Furukawa Y et al. Decreased striatal dopamine D1 receptor-stimulated adenylyl cyclase activity in human methamphetamine users. Am J Psychiatry 2003; 160: 896–903.
Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci 2000; 67: 1507–1515.
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 1997; 386: 830–833.
Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry 2011; 168: 634–641.
Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 2007; 164: 622–629.
Robbins TW, Ersche KD, Everitt BJ . Drug addiction and the memory systems of the brain. Ann N Y Acad Sci 2008; 1141: 1–21.
Yin HH, Ostlund SB, Knowlton BJ, Balleine BW . The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 2005; 22: 513–523.
Belin D, Everitt BJ . Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 2008; 57: 432–441.
Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 2006; 31: 2716–2727.
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage 2008; 39: 1266–1273.
See RE, Elliott JC, Feltenstein MW . The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007; 194: 321–331.
Acknowledgements
The recruitment and psychological screening were carried out at the Harbor University of California—Los Angeles Medical Center and Veteran Administration Medical Center at Portland, Oregon. We thank David Schlyer and Michael Schueller for performing cyclotron operations; Donald Warner, David Alexoff and Paul Vaska for performing PET operations; Richard Ferrieri, Colleen Shea, Youwen Xu, Lisa Muench and Payton King for radiotracer preparation and analysis, Karen Apelskog-Torres for study protocol preparation, and Barbara Hubbard and Pauline Carter for patient care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The PET study was carried out at the Brookhaven National Laboratory with infrastructure support from the US Department of Energy OBER (DE-ACO2-76CH00016) and under support in part by NIH: R01DA06891 (Dr Wang), MO1RR10710 (the General Clinical Research Center of Stony Brook University) and Z01AA000550 (Dr Volkow). The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Wang, G., Smith, L., Volkow, N. et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 17, 918–925 (2012). https://doi.org/10.1038/mp.2011.86
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2011.86
Keywords
This article is cited by
-
PET imaging of dopamine transporters and D2/D3 receptors in female monkeys: effects of chronic cocaine self-administration
Neuropsychopharmacology (2023)
-
Repeated chemogenetic activation of dopaminergic neurons induces reversible changes in baseline and amphetamine-induced behaviors
Psychopharmacology (2023)
-
Dopamine dysfunction in stimulant use disorders: mechanistic comparisons and implications for treatment
Molecular Psychiatry (2022)
-
Prenatal Amphetamine-Induced Dopaminergic Alteration in a Gender- and Estrogen-Dependent Manner
Neurochemical Research (2022)
-
Unpleasant Sound Elicits Negative Emotion and Reinstates Drug Seeking
Molecular Neurobiology (2019)