Abstract
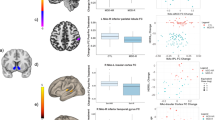
Positive emotionality (PEM) (personality construct of well-being, achievement/motivation, social and closeness) has been associated with striatal dopamine D2 receptor availability in healthy controls. As striatal D2 receptors modulate activity in orbitofrontal cortex (OFC) and cingulate (brain regions that process natural and drug rewards), we hypothesized that these regions underlie PEM. To test this, we assessed the correlation between baseline brain glucose metabolism (measured with positron emission tomography and [18F]fluoro-deoxyglucose) and scores on PEM (obtained from the multidimensional personality questionnaire or MPQ) in healthy controls (n=47). Statistical parametric mapping (SPM) analyses revealed that PEM was positively correlated (Pc<0.05, voxel corrected) with metabolism in various cortical regions that included orbitofrontal (Brodman area, BA 11, 47) and cingulate (BA 23, 32) and other frontal (BA 10, 9), parietal (precuneus, BA 40) and temporal (BA 20, 21) regions that overlap with the brain's default mode network (DMN). Correlations with the other two main MPQ personality dimensions (negative emotionality and constraint) were not significant (SPM Pc<0.05). Our results corroborate an involvement of orbitofrontal and cingulate regions in PEM, which is considered a trait that protects against substance use disorders. As dysfunction of OFC and cingulate is a hallmark of addiction, these findings support a common neural basis underlying protective personality factors and brain dysfunction underlying substance use disorders. In addition, we also uncovered an association between PEM and baseline metabolism in regions from the DMN, which suggests that PEM may relate to global cortical processes that are active during resting conditions (introspection, mind wandering).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Whittle S, Allen NB, Lubman DI, Yücel M . The neurobiological basis of temperament: towards a better understanding of psychopathology. Neurosci Biobehav Rev 2006; 30: 511–525.
Wills TA, Sandy JM, Yaeger A, Shinar O . Family risk factors and adolescent substance use: moderation effects for temperament dimensions. Dev Psychol 2001; 37: 283–297.
Berridge KC, Kringelbach ML . Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008; 199: 457–480.
Depue RA, Collins PF . Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci 1999; 22: 491–569.
Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F . Imaging dopamine's role in drug abuse and addiction. Neuropharmacology 2009; 56 (Suppl 1): 3–8.
Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry 2006; 63: 999–1008.
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry 1999; 156: 1440–1443.
Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse 2002; 46: 79–82.
Thanos PK, Michaelides M, Umegaki H, Volkow ND . D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse 2008; 62: 481–486.
Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW . Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci 2008; 363: 3125–3135.
Goldstein RZ, Volkow ND . Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 2002; 159: 1642–1652.
Kringelbach ML . The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005; 6: 691–702.
Rushworth MF, Behrens TE, Rudebeck PH, Walton ME . Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci 2007; 11: 168–176.
Grabenhorst F, Rolls ET, Parris BA . From affective value to decision-making in the prefrontal cortex. Eur J Neurosci 2008; 28: 1930–1939.
Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD et al. Acute effects of cocaine on human brain activity and emotion. Neuron 1997; 19: 591–611.
Lebreton M, Barnes A, Miettunen J, Peltonen L, Ridler K, Veijola J et al. The brain structural disposition to social interaction. Eur J Neurosci 2009; 29: 2247–2252.
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 1977; 28: 897–916.
Patrick CJ, Curtin JJ, Tellegen A . Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess 2002; 14: 150–163.
Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C et al. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One 2008; 3: e2017.
Wang GJ, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL et al. Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology 1993; 186: 59–65.
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE . Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 1979; 6: 371–388.
Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ . Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2: 189–210.
Worsley K, Marrett S, Neelin P, Vandal A, Friston K, Evans A . A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1996; 4: 58–73.
Talairach J, Tournoux P . Co-planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers: New York, 1988.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L et al. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp 2000; 10: 120–131.
Buckner RL, Andrews-Hanna JR, Schacter DL . The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 2008; 1124: 1–38.
Deckersbach T, Miller KK, Klibanski A, Fischman A, Dougherty DD, Blais MA et al. Regional cerebral brain metabolism correlates of neuroticism and extraversion. Depress Anxiety 2006; 23: 133–138.
Gusnard DA, Ollinger JM, Shulman GL, Cloninger CR, Price JL, Van Essen DC et al. Persistence and brain circuitry. Proc Natl Acad Sci USA 2003; 100: 3479–3484.
Whittle S, Yücel M, Fornito A, Barrett A, Wood SJ, Lubman DI et al. Neuroanatomical correlates of temperament in early adolescents. J Am Acad Child Adolesc Psychiatry 2008; 47: 682–693.
Linke J, Kirsch P, King AV, Gass A, Hennerici MG, Bongers A et al. Motivational orientation modulates the neural response to reward. Neuroimage 2010; 49: 2618–2625.
Roelofs K, Minelli A, Mars RB, van Peer J, Toni I . On the neural control of social emotional behavior. Soc Cogn Affect Neurosci 2009; 4: 50–58.
Reekie YL, Braesicke K, Man MS, Roberts AC . Uncoupling of behavioral and autonomic responses after lesions of the primate orbitofrontal cortex. Proc Natl Acad Sci USA 2008; 105: 9787–9792.
Rolls ET, Hornak J, Wade D, McGrath J . Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry 1994; 57: 1518–1524.
Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P . The anterior congulate cortex: the evolution of an interface between emotion and cognition. Ann New York Acad Sci 2001; 935: 107–117.
Kufahl P, Li Z, Risinger R, Rainey C, Piacentine L, Wu G et al. Expectation modulates human brain responses to acute cocaine: a functional magnetic resonance imaging study. Biol Psychiatry 2008; 63: 222–230.
Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F et al. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend 2007; 87: 233–240.
Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN . Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci 2004; 7: 1370–1375.
Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA 2002; 99: 523–528.
Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA et al. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci USA 2009; 106: 9453–9458.
Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F . Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 2007; 64: 1575–1579.
Volkow ND, Fowler JS . Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 2000; 10: 318–325.
Acton GS . Measurement of impulsivity in a hierarchical model of personality traits: implications for substance use. Subst Use Misuse 2003; 38: 67–83.
Hakamata Y, Iwase M, Iwata H, Kobayashi T, Tamaki T, Nishio M et al. Regional brain cerebral glucose metabolism and temperament: a positron emission tomography study. Neurosci Lett 2006; 396: 33–37.
Youn T, Lyoo IK, Kim JK, Park HJ, Ha KS, Lee DS et al. Relationship between personality trait and regional cerebral glucose metabolism assessed with positron emission tomography. Biol Psychol 2002; 60: 109–120.
Acknowledgements
This research was supported by the National Institutes of Health (Intramural Research Program of the National Institute on Alcoholism and Alcohol Abuse and Grant AA 09481). We thank David Schlyer for cyclotron operations, Colleen Shea and Youwen Xu for radiotracer synthesis, Pauline Carter for nursing care, Karen Apelskog for protocol coordination and Ruben Baler and Linda Thomas for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Volkow, N., Tomasi, D., Wang, GJ. et al. Positive emotionality is associated with baseline metabolism in orbitofrontal cortex and in regions of the default network. Mol Psychiatry 16, 818–825 (2011). https://doi.org/10.1038/mp.2011.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2011.30
Keywords
This article is cited by
-
The effects of nalmefene on the impulsive and reflective system in alcohol use disorder: A resting-state fMRI study
Psychopharmacology (2022)
-
Increased functional connectivity in the resting-state basal ganglia network after acute heroin substitution
Translational Psychiatry (2015)
-
Functional neuroimaging of extraversion-introversion
Neuroscience Bulletin (2015)
-
Brain correlates of pro-social personality traits: a voxel-based morphometry study
Brain Imaging and Behavior (2013)
-
Obesity and Mental Illness: Implications for Cognitive Functioning
Advances in Therapy (2013)