Abstract
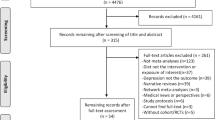
We conducted a meta-analysis of randomized, placebo-controlled trials of omega-3 fatty acid (FA) treatment of major depressive disorder (MDD) in order to determine efficacy and to examine sources of heterogeneity between trials. PubMed (1965-May 2010) was searched for randomized, placebo-controlled trials of omega-3 FAs for MDD. Our primary outcome measure was standardized mean difference in a clinical measure of depression severity. In stratified meta-analysis, we examined the effects of trial duration, trial methodological quality, baseline depression severity, diagnostic indication, dose of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in omega-3 preparations, and whether omega-3 FA was given as monotherapy or augmentation. In 13 randomized, placebo-controlled trials examining the efficacy of omega-3 FAs involving 731 participants, meta-analysis demonstrated no significant benefit of omega-3 FA treatment compared with placebo (standard mean difference (SMD)=0.11, 95% confidence interval (CI): −0.04, 0.26). Meta-analysis demonstrated significant heterogeneity and publication bias. Nearly all evidence of omega-3 benefit was removed after adjusting for publication bias using the trim-and-fill method (SMD=0.01, 95% CI: −0.13, 0.15). Secondary analyses suggested a trend toward increased efficacy of omega-3 FAs in trials of lower methodological quality, trials of shorter duration, trials which utilized completers rather than intention-to-treat analysis, and trials in which study participants had greater baseline depression severity. Current published trials suggest a small, non-significant benefit of omega-3 FAs for major depression. Nearly all of the treatment efficacy observed in the published literature may be attributable to publication bias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Geddes JR, Freemantle N, Mason J, Eccles MP, Boynton J . SSRIs versus other antidepressants for depressive disorder. Cochrane Database Syst Rev 2000; CD001851.
Anderson IM . Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord 2000; 58: 19–36.
Chalon S . Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids 2006; 75: 259–269.
Hibbeln JR . Fish consumption and major depression. Lancet 1998; 351: 1213.
Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamaki H et al. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv 2001; 52: 529–531.
Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H . Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord 1996; 38: 35–46.
Peet M, Murphy B, Shay J, Horrobin D . Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry 1998; 43: 315–319.
Appleton KM, Hayward RC, Gunnell D, Peters TJ, Rogers PJ, Kessler D et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am J Clin Nutr 2006; 84: 1308–1316.
Lin PY, Su KP . A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry 2007; 68: 1056–1061.
Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 2006; 67: 1954–1967.
Appleton KM, Rogers PJ, Ness AR . Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr 2010; 91: 757–770.
Kraguljac NV, Montori VM, Pavuluri M, Chai HS, Wilson BS, Unal SS . Efficacy of omega-3 fatty acids in mood disorders - a systematic review and metaanalysis. Psychopharmacol Bull 2009; 42: 39–54.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J . An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571.
Hamilton M . A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62.
Montgomery SA, Asberg M . A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389.
Higgins J, Green S, (eds). Cochrane Handbook for Systematic Reviews of Interventions 4.2.5. John Wiley & Sons,: Chishester, UK, 2005.
Egger M, Davey Smith G, Schneider M, Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG . Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol 2006; 6: 50.
Angst J, Amrein R, Stabl M . Moclobemide and tricyclic antidepressants in severe depression: meta-analysis and prospective studies. J Clin Psychopharmacol 1985; 15: 16S–26S.
Carmody TJ, Rush AJ, Bernstein I, Warden D, Brannan S, Burnham D et al. The Montgomery Asberg and the Hamilton ratings of depression: a comparison of measures. Eur Neuropsychopharmacol 2006; 16: 601–611.
Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S . Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials 1995; 16: 62–73.
Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A et al. A systematic review of controlled trials of the effectiveness and cost effectiveness of brief psychological treatments for depression. Health Technol Assess 2001; 5: 1–73.
Deeks JJ, Altman DG, Bradburn MJ (eds). Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. BMJ Publication Group: London, 2001.
Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999; 56: 407–412.
Keck Jr PE, Mintz J, McElroy SL, Freeman MP, Suppes T, Frye MA et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry 2006; 60: 1020–1022.
Frangou S, Lewis M, McCrone P . Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry 2006; 188: 46–50.
Fux M, Benjamin J, Nemets B . A placebo-controlled cross-over trial of adjunctive EPA in OCD. J Psychiatr Res 2004; 38: 323–325.
Hallahan B, Hibbeln JR, Davis JM, Garland MR . Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. Br J Psychiatry 2007; 190: 118–122.
Zanarini MC, Frankenburg FR . omega-3 fatty acid treatment of women with borderline personality disorder: a double-blind, placebo-controlled pilot study. Am J Psychiatry 2003; 160: 167–169.
Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 2006; 63: 1402–1408.
Mischoulon D, Best-Popescu C, Laposata M, Merens W, Murakami JL, Wu SL et al. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol 2008; 18: 639–645.
Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH . Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry 2006; 163: 1098–1100.
Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust NZ J Psychiatry 2008; 42: 192–198.
Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS . Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. Jama 2009; 302: 1651–1657.
Peet M, Horrobin DF . A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 2002; 59: 913–919.
Nemets B, Stahl Z, Belmaker RH . Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002; 159: 477–479.
Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2008; 69: 644–651.
Su KP, Huang SY, Chiu CC, Shen WW . Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 2003; 13: 267–271.
Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ . A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 2003; 160: 996–998.
Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA . Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids 2005; 72: 211–218.
Grenyer BF, Crowe T, Meyer B, Owen AJ, Grigonis-Deane EM, Caputi P et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 1393–1396.
Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ . Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord 2008; 110: 142–148.
Rees AM, Austin MP, Parker GB . Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust NZJ Psychiatry 2008; 42: 199–205.
Lucas M, Asselin G, Merette C, Poulin MJ, Dodin S . Effects of ethyl-eicosapentaenoic acid omega-3 fatty acid supplementation on hot flashes and quality of life among middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Menopause 2009; 16: 357–366.
Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry 2009; 70: 1636–1644.
Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr 2008; 99: 421–431.
da Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R et al. Depression in Parkinson's disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord 2008; 111: 351–359.
Sontrop J, Campbell MK . Omega-3 polyunsaturated fatty acids and depression: a review of the evidence and a methodological critique. Prev Med 2006; 42: 4–13.
Appleton KM, Rogers PJ, Ness AR . Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr 2010; 91: 757–770.
Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M . A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry 2001; 158: 2071–2074.
Peet M, Horrobin DF . A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res 2002; 36: 7–18.
Laurin D, Carmichael PH . Combining or not combining published results in the presence of heterogeneity? Am J Clin Nutr. 2010; 92: 669–670; author reply 70–1.
Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. Jama 2010; 303: 47–53.
Fritsche K . Fatty acids as modulators of the immune response. Annu Rev Nutr 2006; 26: 45–73.
Ross BM . Omega-3 fatty acid deficiency in major depressive disorder is caused by the interaction between diet and a genetically determined abnormality in phospholipid metabolism. Med Hypotheses 2007; 68: 515–524.
Covault J, Pettinati H, Moak D, Mueller T, Kranzler HR . Association of a long-chain fatty acid-CoA ligase 4 gene polymorphism with depression and with enhanced niacin-induced dermal erythema. Am J Med Genet B Neuropsychiatr Genet 2004; 127B: 42–47.
Papadimitriou GN, Dikeos DG, Souery D, Del-Favero J, Massat I, Avramopoulos D et al. Genetic association between the phospholipase A2 gene and unipolar affective disorder: a multicentre case-control study. Psychiatr Genet 2003; 13: 211–220.
Pae CU, Yu HS, Kim JJ, Lee CU, Lee SJ, Lee KU et al. BanI polymorphism of the cytosolic phospholipase A2 gene and mood disorders in the Korean population. Neuropsychobiology 2004; 49: 185–188.
Grimble RF, Howell WM, O’Reilly G, Turner SJ, Markovic O, Hirrell S et al. The ability of fish oil to suppress tumor necrosis factor alpha production by peripheral blood mononuclear cells in healthy men is associated with polymorphisms in genes that influence tumor necrosis factor alpha production. Am J Clin Nutr 2002; 76: 454–459.
Sontrop JM, Campbell MK, Evers SE, Speechley KN, Avison WR . Fish consumption among pregnant women in London, Ontario: associations with socio-demographic and health and lifestyle factors. Can J Public Health 2007; 98: 389–394.
Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I . The case of the misleading funnel plot. BMJ 2006; 333: 597–600.
Acknowledgements
We acknowledge the National Institute of Mental Health support of the Yale Child Study Center Research Training Program (MHB), the National Institutes of Health 1K23MH091240-01 (MHB), the APIRE/Eli Lilly Psychiatric Research Fellowship (MHB), the AACAP/Eli Lilly Pilot Research Award (MHB), the Trichotillomania Learning Center (MHB), NARSAD (MHB and JH) and UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research (MHB and JH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bloch, M., Hannestad, J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry 17, 1272–1282 (2012). https://doi.org/10.1038/mp.2011.100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2011.100
Keywords
This article is cited by
-
Interaction of fatty acid quality indices and genes related to lipid homeostasis on mental health among overweight and obese women
Scientific Reports (2023)
-
The role of polyunsaturated fatty acids in the neurobiology of major depressive disorder and suicide risk
Molecular Psychiatry (2023)
-
Efficacy of eicosapentaenoic acid in inflammatory depression: study protocol for a match-mismatch trial
BMC Psychiatry (2022)
-
High-dose omega-3 polyunsaturated fatty acid supplementation might be more superior than low-dose for major depressive disorder in early therapy period: a network meta-analysis
BMC Psychiatry (2020)
-
The efficacy and safety of omega-3 fatty acids on depressive symptoms in perinatal women: a meta-analysis of randomized placebo-controlled trials
Translational Psychiatry (2020)