Abstract
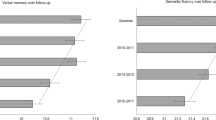
Carriers of the APOE E4 allele have an increased risk of developing Alzheimer's disease. However, it is less clear whether APOE E4 status may also be involved in non-pathological cognitive ageing. The present study investigated the associations between APOE genotypes and cognitive change over 8 years in older community-dwelling individuals. APOE genotype was determined in 501 participants of the Lothian Birth Cohort 1921, whose intelligence had been measured in childhood in the Scottish Mental Survey 1932. A polymorphic variant of TOMM40 (rs10524523) was included to differentiate between the effects of the APOE E3 and E4 allelic variants. Cognitive performance on the domains of verbal memory, abstract reasoning and verbal fluency was assessed at mean age 79 years (n=501), and again at mean ages of 83 (n=284) and 87 (n=187). Using linear mixed models adjusted for demographic variables, vascular risk factors and IQ at age 11 years, possession of the APOE E4 allele was associated with a higher relative rate of cognitive decline over the subsequent 8 years for verbal memory and abstract reasoning. Individuals with the long allelic variant of TOMM40, which is linked to APOE E4, showed similar results. Verbal fluency was not affected by APOE E4 status. APOE E2 status was not associated with change in cognitive performance over 8 years. In non-demented older individuals, possession of the APOE E4 allele predicted a higher rate of cognitive decline on tests of verbal memory and abstract reasoning between 79 and 87 years. Thus, possession of the APOE E4 allele may not only predispose to Alzheimer's disease, but also appears to be a risk factor for non-pathological decline in verbal memory and abstract reasoning in the ninth decade of life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993; 261: 921–923.
Slooter AJ, Cruts M, Kalmijn S, Hofman A, Breteler MM, Van Broeckhoven C et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Arch Neurol 1998; 55: 964–968.
Cosentino S, Scarmeas N, Helzner E, Glymour MM, Brandt J, Albert M et al. APOE ɛ4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology 2008; 70 (Pt 2): 1842–1849.
Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell Jr PC et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 1994; 7: 180–184.
Mahley RW, Rall Jr SC . Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 2000; 1: 507–537.
Smith JD . Apolipoproteins and aging: emerging mechanisms. Ageing Res Rev 2002; 1: 345–365.
Blair CK, Folsom AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E . APOE genotype and cognitive decline in a middle-aged cohort. Neurology 2005; 64: 268–276.
Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE . The role of APOE-ɛ4 in longitudinal cognitive decline: MacArthur studies of successful aging. Neurology 2003; 60: 1077–1081.
Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL et al. Longitudinal modeling of age-related memory decline and the APOE ɛ4 effect. N Engl J Med 2009; 361: 255–263.
Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF et al. Cognitive change and the APOE ɛ4 allele. Nature 2002; 418: 932.
Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF et al. Apolipoprotein e gene variability and cognitive functions at age 79: a follow-up of the Scottish mental survey of 1932. Psychol Aging 2004; 19: 367–371.
Fillenbaum GG, Landerman LR, Blazer DG, Saunders AM, Harris TB, Launer LJ . The relationship of APOE genotype to cognitive functioning in older African-American and Caucasian community residents. J Am Geriatr Soc 2001; 49: 1148–1155.
Luciano M, Gow AJ, Harris SE, Hayward C, Allerhand M, Starr JM et al. Cognitive ability at age 11 and 70 years, information processing speed, and APOE variation: the Lothian Birth Cohort 1936 study. Psychol Aging 2009; 24: 129–138.
Packard CJ, Westendorp RG, Stott DJ, Caslake MJ, Murray HM, Shepherd J et al. Association between apolipoprotein E4 and cognitive decline in elderly adults. J Am Geriatr Soc 2007; 55: 1777–1785.
Luciano M, Gow AJ, Taylor MD, Hayward C, Harris SE, Campbell H et al. Apolipoprotein E is not related to memory abilities at 70 years of age. Behav Genet 2009; 39: 6–14.
Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S . APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology 2007; 21: 1–8.
Deary IJ, Whalley LJ, St Clair D, Breen G, Leaper S, Lemmon H et al. The influence of the ɛ4 allele of the apolipoprotein E gene on childhood IQ, nonverbal reasoning in old age, and lifetime cognitive change. Intelligence 2003; 31: 85–92.
Bunce D, Fratiglioni L, Small BJ, Winblad B, Backman L . APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology 2004; 63: 816–821.
Small BJ, Basun H, Backman L . Three-year changes in cognitive performance as a function of apolipoprotein E genotype: evidence from very old adults without dementia. Psychol Aging 1998; 13: 80–87.
Wisdom NM, Callahan JL, Hawkins KA . The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging 2011; 32: 63–74.
Small BJ, Rosnick CB, Fratiglioni L, Backman L . Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging 2004; 19: 592–600.
Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC . The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol 2004; 86: 130–147.
Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L . The role of APOE ɛ4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA 1999; 282: 40–46.
Slooter AJ, Van Duijn CM, Bots ML, Ott A, Breteler MB, De Voecht J et al. Apolipoprotein E genotype, atherosclerosis, and cognitive decline: the Rotterdam Study. J Neural Transm Suppl 1998; 53: 17–29.
Lai E, Riley J, Purvis I, Roses A . A 4-Mb high-density single nucleotide polymorphism-based map around human APOE. Genomics 1998; 54: 31–38.
Lutz MW, Crenshaw DG, Saunders AM, Roses AD . Genetic variation at a single locus and age of onset for Alzheimer's disease. Alzheimers Dement 2010; 6: 125–131.
Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer's disease. Pharmacogenomics J 2009; 10: 375–384.
Scottish Council for Research in Education. The intelligence of Scottish children: A national survey of an age-group. Scottish Council for Research in Education: London, 1933.
Deary IJ, Whalley LJ, Starr JM . A lifetime of intelligence: Follow-up studies of the Scottish Mental Surveys of 1932 and 1947. American Psychological Association: Washington, DC, 2009.
Gow AJ, Johnson W, Pattie A, Brett CE, Roberts B, Starr JM et al. Stability and change in intelligence from age 11 to ages 70, 79 and 87: the Lothian Birth Cohorts of 1921 and 1936. Psychol Aging 2010, E-pub ahead of print 25 October 2010; doi: 2010.1037/a0021072.
Gow AJ, Johnson W, Pattie A, Whiteman MC, Starr J, Deary IJ . Mental ability in childhood and cognitive aging. Gerontology 2008; 54: 177–186.
Folstein MF, Folstein SE, McHugh PR . Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198.
Lezak MD . Neuropsychological testing. Oxford University Press: Oxford, England, 1995.
Wechsler D . Wechsler Memory Scale—Revised. Psychological Corporation: New York, 1987.
Raven JC, Court Jr JH . Manual for Raven's Progressive Matrices and Vocabulary Scales. HK Lewis: London, 1977.
Wenham PR, Price WH, Blandell G . Apolipoprotein E genotyping by one-stage PCR. Lancet 1991; 337: 1158–1159.
Verbeke G, Molenberghs G . Linear mixed models for longitudinal data. Springer: New York, 2000.
Den Heijer T, Oudkerk M, Launer LJ, Van Duijn CM, Hofman A, Breteler MM . Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology 2002; 59: 746–748.
Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM . APOE ɛ4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 2010; 67: 93–98.
Melrose RJ, Poulin RM, Stern CE . An fMRI investigation of the role of the basal ganglia in reasoning. Brain Res 2007; 1142: 146–158.
Zimmerman ME, Pan JW, Hetherington HP, Katz MJ, Verghese J, Buschke H et al. Hippocampal neurochemistry, neuromorphometry, and verbal memory in nondemented older adults. Neurology 2008; 70: 1594–1600.
Reitz C, Brickman AM, Brown TR, Manly J, DeCarli C, Small SA et al. Linking hippocampal structure and function to memory performance in an aging population. Arch Neurol 2009; 66: 1385–1392.
Cumming AM, Robertson FW . Polymorphism at the apoprotein-E locus in relation to risk of coronary disease. Clin Genet 1984; 25: 310–313.
Acknowledgements
We thank the LBC1921 participants. We thank Beverly Roberts and Martha Whiteman for data collection. We thank the Scottish Council for Research in Education for allowing access to the Scottish Mental Survey 1932. The Biotechnology and Biological Sciences Research Council funded data collection for Wave 1; a Royal Society-Wolfson Research Merit Award to IJD funded data collection for Wave 2; a grant from the Scottish Government's Health Directorates Chief Scientist Office supported data collection in Wave 3. The work was undertaken by The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (G0700704/84698). Funding from the Biotechnology and Biological Sciences Research Council (BBSRC), Engineering and Physical Sciences Research Council (EPSRC), Economic and Social Research Council (ESRC) and Medical Research Council (MRC) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Schiepers, O., Harris, S., Gow, A. et al. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry 17, 315–324 (2012). https://doi.org/10.1038/mp.2010.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2010.137
Keywords
This article is cited by
-
The effects of a moderate physical activity intervention on physical fitness and cognition in healthy elderly with low levels of physical activity: a randomized controlled trial
Alzheimer's Research & Therapy (2023)
-
Predictors of longitudinal cognitive ageing from age 70 to 82 including APOE e4 status, early-life and lifestyle factors: the Lothian Birth Cohort 1936
Molecular Psychiatry (2023)
-
Genetic variation, brain, and intelligence differences
Molecular Psychiatry (2022)
-
Apolipoprotein E ɛ4–related effects on cognition are limited to the Alzheimer’s disease spectrum
GeroScience (2022)
-
Polygenic predictors of age-related decline in cognitive ability
Molecular Psychiatry (2020)