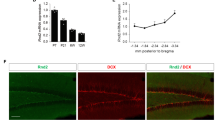
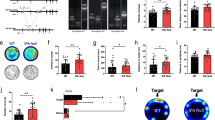
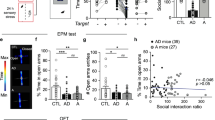
Abstract
Adult hippocampal neurogenesis is a unique example of structural plasticity, the functional role of which has been a matter of intense debate. New transgenic models have recently shown that neurogenesis participates in hippocampus-mediated learning. Here, we show that transgenic animals, in which adult hippocampal neurogenesis has been specifically impaired, exhibit a striking increase in anxiety-related behaviors. Our results indicate that neurogenesis plays an important role in the regulation of affective states and could be the target of new treatments for anxiety disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abrous DN, Koehl M, Le Moal M . Adult neurogenesis: from precursors to network and physiology. Physiol Rev 2005; 85: 523–569.
Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA et al. Neurogenesis in the adult human hippocampus. Nat Med 1998; 4: 1313–1317.
Abrous DN, Wojtowicz JM . Neurogenesis and hippocampal memory system. Cold Spring Harbor Laboratory Press: New York, 2008, 445–461.
Leuner B, Gould E, Shors TJ . Is there a link between adult neurogenesis and learning? Hippocampus 2006; 16: 216–224.
Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P et al. Spatial relational memory requires hippocampal adult neurogenesis. PlosOne 2008; 3: e1959.
Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 2008; 11: 1153–1161.
Zhang CL, Zou Y, He W, Gage FH, Evans RM . A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 2008; 451: 1004–1007
MacLEAN PD . Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosom Med 1949; 11: 338–353.
Papez JW . A proposed mechanism for emotion. Arch Neurol Psychiatry 1937; 79: 237–244.
Bannerman DM, Matthews P, Deacon RM, Rawlins JN . Medial septal lesions mimic effects of both selective dorsal and ventral hippocampal lesions. Behav Neurosci 2004; 118: 1033–1041.
Gray JA . The Neuropsychology of Anxiety. Clarendon Press: Oxford, 1982.
Kessler RC, Birnbaum H, Demler O, Falloon IR, Gagnon E, Guyer M et al. The prevalence and correlates of nonaffective psychosis in the National Comorbidity Survey Replication (NCS-R). Biol Psychiatry 2005; 58: 668–676.
Mineka S, Watson D, Clark LA . Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol 1998; 49: 377–412.
Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 2008; 59: 399–412.
Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER . An animal model of a behavioral intervention for depression. Neuron 2008; 60: 149–161.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–809.
Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 2008; 64: 293–301.
Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G . Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience 2003; 119: 635–642.
Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 2004; 162: 39–47.
Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA 2006; 103: 17501–17506.
Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E . Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 2002; 12: 578–584.
Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA . Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci 1999; 19: 9550–9556.
Lister RG . Ethologically-based animal models of anxiety disorders. Pharmacol Ther 1990; 46: 321–340.
Rodgers RJ, Johnson NJ . Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav 1995; 52: 297–303.
Yang M, Augustsson H, Markham CM, Hubbard DT, Webster D, Wall PM et al. The rat exposure test: a model of mouse defensive behaviors. Physiol Behav 2004; 81: 465–473.
Dupret D, Fabre A, Döbrössy MD, Panatier A, Rodríguez JJ, Lamarque S et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLOS Biology 2007; 5: 1683–1694.
Rao MS, Shetty AK . Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 2004; 19: 234–246.
Sholl DA . Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 1953; 87: 387–406.
Kokoeva MV, Yin H, Flier JS . Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 2005; 310: 679–683.
Belzung C, El Hage W, Moindrot N, Griebel G . Behavioral and neurochemical changes following predatory stress in mice. Neuropharmacology 2001; 41: 400–408.
Ageta H, Murayama A, Migishima R, Kida S, Tsuchida K, Yokoyama M et al. Activin in the brain modulates anxiety-related behavior and adult neurogenesis. PLoS ONE 2008; 3: e1869.
Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M . Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci USA 2008; 105: 15570–15575.
Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci 1999; 2: 833–839.
McNaughton N . Cognitive dysfunction resulting from hippocampal hyperactivity-a possible cause of anxiety disorder? Pharmacol Biochem Behav 1997; 56: 603–611.
Tsetsenis T, Ma XH, Lo IL, Beck SG, Gross C . Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci 2007; 10: 896–902.
Acknowledgements
The authors are grateful to Dr F Chaouloff for his helpful comments. The technical help of Mr C Dupuy, Miss S Lamarque, Miss D Gonzales, and Mrs V Roullot–Lacarrière is greatly acknowledged. This work was financially supported by INSERM and Agence Nationale pour la Recherche (ANR to DNA). CFR was funded by the ‘Ministère délégué à l’Enseignement Supérieur et à la Recherche' and NG by ANR.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary information
Rights and permissions
About this article
Cite this article
Revest, JM., Dupret, D., Koehl, M. et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry 14, 959–967 (2009). https://doi.org/10.1038/mp.2009.15
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2009.15
Keywords
This article is cited by
-
Glial-restricted precursors stimulate endogenous cytogenesis and effectively recover emotional deficits in a model of cytogenesis ablation
Molecular Psychiatry (2024)
-
Transmembrane protein 184B (TMEM184B) promotes expression of synaptic gene networks in the mouse hippocampus
BMC Genomics (2023)
-
Neural underpinnings of open-label placebo effects in emotional distress
Neuropsychopharmacology (2023)
-
Hippocampus: Molecular, Cellular, and Circuit Features in Anxiety
Neuroscience Bulletin (2023)
-
Investigation of effects of transferrin-conjugated gold nanoparticles on hippocampal neuronal activity and anxiety behavior in mice
Molecular and Cellular Biochemistry (2023)