Abstract
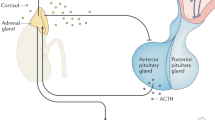
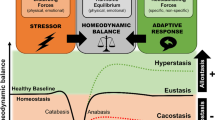
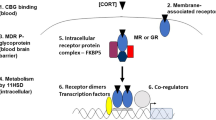
A concatenation of findings from preclinical and clinical studies support a preeminent function for the corticotropin-releasing factor (CRF) system in mediating the physiological response to external stressors and in the pathophysiology of anxiety and depression. Recently, human genetic studies have provided considerable support to several long-standing hypotheses of mood and anxiety disorders, including the CRF hypothesis. These data, reviewed in this report, are congruent with the hypothesis that this system is of paramount importance in mediating stress-related psychopathology. More specifically, variants in the gene encoding the CRF1 receptor interact with adverse environmental factors to predict risk for stress-related psychiatric disorders. In-depth characterization of these variants will likely be important in furthering our understanding of the long-term consequences of adverse experience.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vale W, Spiess J, Rivier C, Rivier J . Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981; 213: 1394–1397.
Britton DR, Koob GF, Rivier J, Vale W . Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci 1982; 31: 363–367.
Kalin NH . Behavioral effects of ovine corticotropin-releasing factor administered to rhesus monkeys. Fed Proc 1985; 44 (1 Part 2): 249–253.
Kalin NH, Shelton SE, Kraemer GW, McKinney WT . Corticotropin-releasing factor administered intraventricularly to rhesus monkeys. Peptides 1983; 4: 217–220.
Koob GF, Bloom FE . Corticotropin-releasing factor and behavior. Fed Proc 1985; 44 (1 Part 2): 259–263.
Koob GF, Thatcher-Britton K . Stimulant and anxiogenic effects of corticotropin releasing factor. Prog Clin Biol Res 1985; 192: 499–506.
Sirinathsinghji DJ, Rees LH, Rivier J, Vale W . Corticotropin-releasing factor is a potent inhibitor of sexual receptivity in the female rat. Nature 1983; 305: 232–235.
Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 1984; 226: 1342–1344.
Nemeroff CB . The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry 1996; 1: 336–342.
Reul JM, Holsboer F . Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol 2002; 2: 23–33.
Nemeroff CB, Vale WW . The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry 2005; 66 (Suppl 7): 5–13.
Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM . International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev 2003; 55: 21–26.
Boorse GC, Denver RJ . Widespread tissue distribution and diverse functions of corticotropin-releasing factor and related peptides. Gen Comp Endocrinol 2006; 146: 9–18.
Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K et al. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA 1994; 91: 8777–8781.
Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, Rivier JE et al. Cloning and characterization of human urocortin. Endocrinology 1996; 137: 3896.
Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 2001; 98: 7570–7575.
Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA 2001; 98: 2843–2848.
Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 1995; 378: 287–292.
Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE . Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol 1999; 415: 285–312.
Wong ML, al-Shekhlee A, Bongiorno PB, Esposito A, Khatri P, Sternberg EM et al. Localization of urocortin messenger RNA in rat brain and pituitary. Mol Psychiatry 1996; 1: 307–312.
Kageyama K, Bradbury MJ, Zhao L, Blount AL, Vale WW . Urocortin messenger ribonucleic acid: tissue distribution in the rat and regulation in thymus by lipopolysaccharide and glucocorticoids. Endocrinology 1999; 140: 5651–5658.
Hashimoto K, Nishiyama M, Tanaka Y, Noguchi T, Asaba K, Hossein PN et al. Urocortins and corticotropin releasing factor type 2 receptors in the hypothalamus and the cardiovascular system. Peptides 2004; 25: 1711–1721.
Chalmers DT, Lovenberg TW, De Souza EB . Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci 1995; 15: 6340–6350.
Hiroi N, Wong ML, Licinio J, Park C, Young M, Gold PW et al. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry 2001; 6: 540–546.
Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA 1995; 92: 836–840.
Sanchez MM, Young LJ, Plotsky PM, Insel TR . Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol 1999; 408: 365–377.
Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW . Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol 1995; 16: 362–382.
Seasholtz AF, Valverde RA, Denver RJ . Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammals. J Endocrinol 2002; 175: 89–97.
Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW . Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature 1991; 349: 423–426.
Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW . The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci USA 1992; 89: 4192–4196.
Vitoratos N, Papatheodorou DC, Kalantaridou SN, Mastorakos G . ‘Reproductive’ corticotropin-releasing hormone. Ann NY Acad Sci 2006; 1092: 310–318.
Valentino RJ, Foote SL, Aston-Jones G . Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res 1983; 270: 363–367.
Tsatsanis C, Dermitzaki E, Venihaki M, Chatzaki E, Minas V, Gravanis A et al. The corticotropin-releasing factor (CRF) family of peptides as local modulators of adrenal function. Cell Mol Life Sci 2007; 64: 1638–1655.
Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB . The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999; 160: 1–12.
Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT . Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept 1997; 71: 15–21.
Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 2000; 24: 410–414.
Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 2000; 24: 403–409.
Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F et al. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet 2000; 24: 415–419.
Sekino A, Ohata H, Mano-Otagiri A, Arai K, Shibasaki T . Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. Psychopharmacology (Berl) 2004; 176: 30–38.
Valdez GR, Sabino V, Koob GF . Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res 2004; 28: 865–872.
Henry B, Vale W, Markou A . The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci 2006; 26: 9142–9152.
Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP . Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety. Brain Res 2001; 902: 135–142.
Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF . Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res 2003; 980: 206–212.
Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC et al. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther 2007; 323: 846–854.
Preil J, Muller MB, Gesing A, Reul JM, Sillaber I, van Gaalen MM et al. Regulation of the hypothalamic-pituitary-adrenocortical system in mice deficient for CRH receptors 1 and 2. Endocrinology 2001; 142: 4946–4955.
Hauger RL, Risbrough V, Brauns O, Dautzenberg FM . Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets 2006; 5: 453–479.
Dunn AJ, Berridge CW . Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev 1990; 15: 71–100.
Keck ME . Corticotropin-releasing factor, vasopressin and receptor systems in depression and anxiety. Amino Acids 2006; 31: 241–250.
Keck ME, Holsboer F, Muller MB . Mouse mutants for the study of corticotropin-releasing hormone receptor function: development of novel treatment strategies for mood disorders. Ann NY Acad Sci 2004; 1018: 445–457.
Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M et al. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol Psychiatry 2008; 13: 1028–1042.
Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci 2003; 6: 1100–1107.
Kageyama K, Suda T . Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus. Endocr J 2009; 56: 335–344.
Schulkin J, Gold PW, McEwen BS . Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology 1998; 23: 219–243.
Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ et al. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry 2009; 14: 37–50.
Banki CM, Bissette G, Arato M, O'Connor L, Nemeroff CB . CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry 1987; 144: 873–877.
Hartline KM, Owens MJ, Nemeroff CB . Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Ann NY Acad Sci 1996; 780: 96–105.
Arato M, Banki CM, Bissette G, Nemeroff CB . Elevated CSF CRF in suicide victims. Biol Psychiatry 1989; 25: 355–359.
Nemeroff CB, Bissette G, Akil H, Fink M . Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy. Corticotrophin-releasing factor, beta-endorphin and somatostatin. Br J Psychiatry 1991; 158: 59–63.
De Bellis MD, Gold PW, Geracioti Jr TD, Listwak SJ, Kling MA . Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry 1993; 150: 656–657.
Veith RC, Lewis N, Langohr JI, Murburg MM, Ashleigh EA, Castillo S et al. Effect of desipramine on cerebrospinal fluid concentrations of corticotropin-releasing factor in human subjects. Psychiatry Res 1993; 46: 1–8.
Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J et al. Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety 1998; 8: 71–79.
Banki CM, Karmacsi L, Bissette G, Nemeroff CB . CSF corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. Eur Neuropsychopharmacol 1992; 2: 107–113.
Austin MC, Janosky JE, Murphy HA . Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry 2003; 8: 324–332.
Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G et al. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry 2006; 59: 594–602.
Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF . Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 1994; 60: 436–444.
Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G . Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology 2003; 28: 1328–1335.
Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 2004; 24: 1478–1485.
Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M . Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 1988; 45: 577–579.
Herringa RJ, Roseboom PH, Kalin NH . Decreased amygdala CRF-binding protein mRNA in post-mortem tissue from male but not female bipolar and schizophrenic subjects. Neuropsychopharmacology 2006; 31: 1822–1831.
Kozicz T, Tilburg-Ouwens D, Faludi G, Palkovits M, Roubos E . Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience 2008; 152: 1015–1023.
Kunzel HE, Zobel AW, Nickel T, Ackl N, Uhr M, Sonntag A et al. Treatment of depression with the CRH-1-receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res 2003; 37: 525–533.
Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 2000; 34: 171–181.
Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T . A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry 2008; 165: 617–620.
Edwards VJ, Holden GW, Felitti VJ, Anda RF . Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry 2003; 160: 1453–1460.
Nemeroff CB . Neurobiological consequences of childhood trauma. J Clin Psychiatry 2004; 65 (Suppl 1): 18–28.
Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci USA 1996; 93: 1619–1623.
Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol Psychiatry 2005; 57: 373–381.
Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM . Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res 2000; 122: 81–103.
Plotsky PM, Meaney MJ . Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res 1993; 18: 195–200.
Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ . Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology 2005; 30: 2192–2204.
Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology 2004; 29: 777–784.
Lee R, Geracioti Jr TD, Kasckow JW, Coccaro EF . Childhood trauma and personality disorder: positive correlation with adult CSF corticotropin-releasing factor concentrations. Am J Psychiatry 2005; 162: 995–997.
Lee RJ, Gollan J, Kasckow J, Geracioti T, Coccaro EF . CSF corticotropin-releasing factor in personality disorder: relationship with self-reported parental care. Neuropsychopharmacology 2006; 31: 2289–2295.
Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB . The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry 2008; 63: 398–405.
Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB . Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry 2001; 158: 575–581.
Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000; 284: 592–597.
Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry 2008; 63: 1147–1154.
Mathew SJ, Price RB, Charney DS . Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet C Semin Med Genet 2008; 148: 89–98.
Risbrough VB, Stein MB . Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav 2006; 50: 550–561.
Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997; 154: 624–629.
Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L et al. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry 2003; 54: 1382–1388.
Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999; 156: 585–588.
Banki CM, Karmacsi L, Bissette G, Nemeroff CB . Cerebrospinal fluid neuropeptides in mood disorder and dementia. J Affect Disord 1992; 25: 39–45.
Fossey MD, Lydiard RB, Ballenger JC, Laraia MT, Bissette G, Nemeroff CB . Cerebrospinal fluid corticotropin-releasing factor concentrations in patients with anxiety disorders and normal comparison subjects. Biol Psychiatry 1996; 39: 703–707.
Jolkkonen J, Lepola U, Bissette G, Nemeroff C, Riekkinen P . CSF corticotropin-releasing factor is not affected in panic disorder. Biol Psychiatry 1993; 33: 136–138.
Heilig M, Koob GF . A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 2007; 30: 399–406.
Valdez GR, Koob GF . Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav 2004; 79: 671–689.
Adinoff B, Anton R, Linnoila M, Guidotti A, Nemeroff CB, Bissette G . Cerebrospinal fluid concentrations of corticotropin-releasing hormone (CRH) and diazepam-binding inhibitor (DBI) during alcohol withdrawal and abstinence. Neuropsychopharmacology 1996; 15: 288–295.
Hawley RJ, Nemeroff CB, Bissette G, Guidotti A, Rawlings R, Linnoila M . Neurochemical correlates of sympathetic activation during severe alcohol withdrawal. Alcohol Clin Exp Res 1994; 18: 1312–1316.
Skelton KH, Nemeroff CB, Knight DL, Owens MJ . Chronic administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. J Neurosci 2000; 20: 1240–1248.
Heinrichs SC, Koob GF . Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther 2004; 311: 427–440.
Kendler KS . Twin studies of psychiatric illness. Current status and future directions. Arch Gen Psychiatry 1993; 50: 905–915.
Kendler KS . Genetic epidemiology in psychiatry. Taking both genes and environment seriously. Arch Gen Psychiatry 1995; 52: 895–899.
Kendler KS, Gatz M, Gardner CO, Pedersen NL . A Swedish national twin study of lifetime major depression. Am J Psychiatry 2006; 163: 109–114.
Hettema JM, Neale MC, Kendler KS . A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry 2001; 158: 1568–1578.
Sullivan PF, Neale MC, Kendler KS . Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000; 157: 1552–1562.
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J et al. Initial sequencing and analysis of the human genome. Nature 2001; 409: 860–921.
Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG et al. The sequence of the human genome. Science 2001; 291: 1304–1351.
McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW et al. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet 2005; 77: 582–595.
Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger Jr JI et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: bipolar disorder. Am J Hum Genet 2003; 73: 49–62.
Levinson DF . The genetics of depression: a review. Biol Psychiatry 2006; 60: 84–92.
Smoller JW, Gardner-Schuster E, Covino J . The genetic basis of panic and phobic anxiety disorders. Am J Med Genet C Semin Med Genet 2008; 148: 118–126.
Stratakis CA, Sarlis NJ, Berrettini WH, Badner JA, Chrousos GP, Gershon ES et al. Lack of linkage between the corticotropin-releasing hormone (CRH) gene and bipolar affective disorder. Mol Psychiatry 1997; 2: 483–485.
Liu J, Juo SH, Dewan A, Grunn A, Tong X, Brito M et al. Evidence for a putative bipolar disorder locus on 2p13–16 and other potential loci on 4q31, 7q34, 8q13, 9q31, 10q21–24, 13q32, 14q21 and 17q11–12. Mol Psychiatry 2003; 8: 333–342.
Marcheco-Teruel B, Flint TJ, Wikman FP, Torralbas M, Gonzalez L, Blanco L et al. A genome-wide linkage search for bipolar disorder susceptibility loci in a large and complex pedigree from the eastern part of Cuba. Am J Med Genet B Neuropsychiatr Genet 2006; 141B: 833–843.
Fanous AH, Neale MC, Webb BT, Straub RE, O'Neill FA, Walsh D et al. Novel linkage to chromosome 20p using latent classes of psychotic illness in 270 Irish high-density families. Biol Psychiatry 2008; 64: 121–127.
Kelsoe JR, Spence MA, Loetscher E, Foguet M, Sadovnick AD, Remick RA et al. A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc Natl Acad Sci USA 2001; 98: 585–590.
Camp NJ, Lowry MR, Richards RL, Plenk AM, Carter C, Hensel CH et al. Genome-wide linkage analyses of extended Utah pedigrees identifies loci that influence recurrent, early-onset major depression and anxiety disorders. Am J Med Genet B Neuropsychiatr Genet 2005; 135B: 85–93.
Hanna GL, Veenstra-Vanderweele J, Cox NJ, Van Etten M, Fischer DJ, Himle JA et al. Evidence for a susceptibility locus on chromosome 10p15 in early-onset obsessive-compulsive disorder. Biol Psychiatry 2007; 62: 856–862.
Risch N, Merikangas K . The future of genetic studies of complex human diseases. Science 1996; 273: 1516–1517.
Brookes AJ . The essence of SNPs. Gene 1999; 234: 177–186.
Ragoussis J . Genotyping technologies for genetic research. Annu Rev Genomics Hum Genet 2009; 10: 117–133.
International HapMap Consortium. The International HapMap Project. Nature 2003; 426: 789–796.
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007; 447: 661–678.
Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry 2008; 13: 197–207.
Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K et al. Whole-genome association study of bipolar disorder. Mol Psychiatry 2008; 13: 558–569.
Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008; 40: 1056–1058.
Schulze TG, Detera-Wadleigh SD, Akula N, Gupta A, Kassem L, Steele J et al. Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Mol Psychiatry 2009; 14: 487–491.
Muglia P, Tozzi F, Galwey NW, Francks C, Upmanyu R, Kong XQ et al. Genome-wide association study of recurrent major depressive disorder in two European case-control cohorts. Mol Psychiatry 2008 Dec 23, e-pub ahead of print.
Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry 2009; 14: 359–375.
Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 2007; 449: 851–861.
Shimmin LC, Natarajan S, Ibarguen H, Montasser M, Kim DK, Hanis CL et al. Corticotropin releasing hormone (CRH) gene variation: comprehensive resequencing for variant and molecular haplotype discovery in monosomic hybrid cell lines. DNA Seq 2007; 18: 434–444.
Baerwald CG, Mok CC, Fife MS, Tikly M, Lau CS, Wordsworth BP et al. Distribution of corticotropin-releasing hormone promoter polymorphism in different ethnic groups: evidence for natural selection in human populations. Immunogenetics 1999; 49: 894–899.
Baerwald CG, Panayi GS, Lanchbury JS . A new XmnI polymorphism in the regulatory region of the corticotropin releasing hormone gene. Hum Genet 1996; 97: 697–698.
Wagner U, Wahle M, Moritz F, Hantzschel H, Baerwald CG . Promoter polymorphisms regulating corticotrophin-releasing hormone transcription in vitro. Horm Metab Res 2006; 38: 69–75.
Wagner U, Wahle M, Malysheva O, Hantzschel H, Baerwald C . Sequence variants of the CRH 5′-flanking region: effects on DNA-protein interactions studied by EMSA in PC12 cells. Ann NY Acad Sci 2006; 1069: 20–33.
Rosmond R, Chagnon M, Bouchard C, Bjorntorp P . A polymorphism in the regulatory region of the corticotropin-releasing hormone gene in relation to cortisol secretion, obesity, and gene-gene interaction. Metabolism 2001; 50: 1059–1062.
Kyllo JH, Collins MM, Vetter KL, Cuttler L, Rosenfield RL, Donohoue PA . Linkage of congenital isolated adrenocorticotropic hormone deficiency to the corticotropin releasing hormone locus using simple sequence repeat polymorphisms. Am J Med Genet 1996; 62: 262–267.
Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X et al. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry 2008; 65: 934–944.
Gu J, Sadler L, Daiger S, Wells D, Wagner M . Dinucleotide repeat polymorphism at the CRH gene. Hum Mol Genet 1993; 2: 85.
Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR et al. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry 2003; 54: 1376–1381.
Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM et al. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol Psychiatry 2005; 57: 1485–1492.
Keck ME, Kern N, Erhardt A, Unschuld PG, Ising M, Salyakina D et al. Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. Am J Med Genet B Neuropsychiatr Genet 2008; 147B: 1196–1204.
Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet 2004; 36: 1319–1325.
Wasserman D, Sokolowski M, Rozanov V, Wasserman J . The CRHR1 gene: a marker for suicidality in depressed males exposed to low stress. Genes Brain Behav 2008; 7: 14–19.
Binder EB, Owens MJ, Liu W, Deveau TC, Rush AJ, Trivedi M et al. Association of polymorphisms in genes regulating the CRF system with antidepressant treatment response in the STAR*D sample. Arch Gen Psychiatry 2009 (in press).
Claes S, Villafuerte S, Forsgren T, Sluijs S, Del-Favero J, Adolfsson R et al. The corticotropin-releasing hormone binding protein is associated with major depression in a population from Northern Sweden. Biol Psychiatry 2003; 54: 867–872.
Van Den Eede F, Venken T, Del-Favero J, Norrback KF, Souery D, Nilsson LG et al. Single nucleotide polymorphism analysis of corticotropin-releasing factor-binding protein gene in recurrent major depressive disorder. Psychiatry Res 2007; 153: 17–25.
Van Den Eede F, Venken T, Van Den Bogaert A, Del-Favero J, Norrback KF, Nilsson LG et al. Single nucleotide polymorphism analysis of corticotropin-releasing factor-binding protein gene in bipolar disorder. Psychiatr Genet 2007; 17: 304–307.
Enoch MA, Shen PH, Ducci F, Yuan Q, Liu J, White KV et al. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS One 2008; 3: e3620.
Catalano RD, Kyriakou T, Chen J, Easton A, Hillhouse EW . Regulation of corticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: identification of multiple splice variants. Mol Endocrinol 2003; 17: 395–410.
Ardati A, Goetschy V, Gottowick J, Henriot S, Valdenaire O, Deuschle U et al. Human CRF2 alpha and beta splice variants: pharmacological characterization using radioligand binding and a luciferase gene expression assay. Neuropharmacology 1999; 38: 441–448.
Kostich WA, Chen A, Sperle K, Largent BL . Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol 1998; 12: 1077–1085.
Valdenaire O, Giller T, Breu V, Gottowik J, Kilpatrick G . A new functional isoform of the human CRF2 receptor for corticotropin-releasing factor. Biochim Biophys Acta 1997; 1352: 129–132.
Nanda SA, Roseboom PH, Nash GA, Speers JM, Kalin NH . Characterization of the human corticotropin-releasing factor2(a) receptor promoter: regulation by glucocorticoids and the cyclic adenosine 5′-monophosphate pathway. Endocrinology 2004; 145: 5605–5615.
Tochigi M, Kato C, Otowa T, Hibino H, Marui T, Ohtani T et al. Association between corticotropin-releasing hormone receptor 2 (CRHR2) gene polymorphism and personality traits. Psychiatry Clin Neurosci 2006; 60: 524–526.
De Luca V, Tharmalingam S, Kennedy JL . Association study between the corticotropin-releasing hormone receptor 2 gene and suicidality in bipolar disorder. Eur Psychiatry 2007; 22: 282–287.
Papiol S, Arias B, Gasto C, Gutierrez B, Catalan R, Fananas L . Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Affect Disord 2007; 104: 83–90.
Tharmalingam S, King N, De Luca V, Rothe C, Koszycki D, Bradwejn J et al. Lack of association between the corticotrophin-releasing hormone receptor 2 gene and panic disorder. Psychiatr Genet 2006; 16: 93–97.
Villafuerte SM, Del-Favero J, Adolfsson R, Souery D, Massat I, Mendlewicz J et al. Gene-based SNP genetic association study of the corticotropin-releasing hormone receptor-2 (CRHR2) in major depression. Am J Med Genet 2002; 114: 222–226.
Grammatopoulos DK, Chrousos GP . Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab 2002; 13: 436–444.
Pisarchik A, Slominski A . Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur J Biochem 2004; 271: 2821–2830.
Ross PC, Kostas CM, Ramabhadran TV . A variant of the human corticotropin-releasing factor (CRF) receptor: cloning, expression and pharmacology. Biochem Biophys Res Commun 1994; 205: 1836–1842.
Pisarchik A, Slominski AT . Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J 2001; 15: 2754–2756.
Jin D, He P, You X, Zhu X, Dai L, He Q et al. Expression of corticotropin-releasing hormone receptor type 1 and type 2 in human pregnant myometrium. Reprod Sci 2007; 14: 568–577.
Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, Barnard J et al. A common inversion under selection in Europeans. Nat Genet 2005; 37: 129–137.
Koolen DA, Vissers LE, Pfundt R, de Leeuw N, Knight SJ, Regan R et al. A new chromosome 17q21.31 microdeletion syndrome associated with a common inversion polymorphism. Nat Genet 2006; 38: 999–1001.
Sharp AJ, Hansen S, Selzer RR, Cheng Z, Regan R, Hurst JA et al. Discovery of previously unidentified genomic disorders from the duplication architecture of the human genome. Nat Genet 2006; 38: 1038–1042.
Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S et al. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat Genet 2006; 38: 1032–1037.
Tan TY, Aftimos S, Worgan L, Susman R, Wilson M, Ghedia S et al. Phenotypic expansion and further characterization of the 17q21.31 microdeletion syndrome. J Med Genet 2009; 46: 480–489.
Tan TY, Aftimos S, Worgan L, Susman R, Wilson M, Ghedia S et al. Phenotypic expansion and further characterisation of the 17q21.31 microdeletion syndrome. J Med Genet 2009; 46: 480–489.
Kirchhoff M, Bisgaard AM, Duno M, Hansen FJ, Schwartz M . A 17q21.31 microduplication, reciprocal to the newly described 17q21.31 microdeletion, in a girl with severe psychomotor developmental delay and dysmorphic craniofacial features. Eur J Med Genet 2007; 50: 256–263.
Hodges LM, Weissman MM, Haghighi F, Costa R, Bravo O, Evgrafov O et al. Association and linkage analysis of candidate genes GRP, GRPR, CRHR1, and TACR1 in panic disorder. Am J Med Genet B Neuropsychiatr Genet 2009; 150B: 65–73.
Licinio J, O'Kirwan F, Irizarry K, Merriman B, Thakur S, Jepson R et al. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry 2004; 9: 1075–1082.
Liu Z, Zhu F, Wang G, Xiao Z, Tang J, Liu W et al. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett 2007; 414: 155–158.
Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J et al. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett 2006; 404: 358–362.
Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry 2008; 65: 190–200.
Wasserman D, Sokolowski M, Rozanov V, Wasserman J . The CRHR1 gene: a marker for suicidality in depressed males exposed to low stress. Genes Brain Behav 2008; 7: 14–19.
Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry 2009; 66: 978–985.
Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL . Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry 2009; 66: 681–685.
Heim CM, Bradley B, Mletzko T, Deveau TC, Musselman DL, Nemeroff CB et al. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Front Behav Neurosci 2009 (in press).
Ressler KJ, Bradley B, Mercer KB, Deveau TC, Smith AK, Gillespie CF et al. Polymorphisms in CRHR1 and the serotonin transporter loci: gene × gene × environment interactions on depressive symptoms. Am J Med Genet B Psychiatric Genet 2009 (in press).
Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M . Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry 2008; 63: 146–151.
Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH et al. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol 2009; 17: 1–12.
Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML et al. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci USA 2009; 106: 14593–14598.
Derijk RH, de Kloet ER . Corticosteroid receptor polymorphisms: determinants of vulnerability and resilience. Eur J Pharmacol 2008; 583: 303–311.
Binder EB . The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009; 34: 99–109.
Mueller BR, Bale TL . Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci 2008; 28: 9055–9065.
Szyf M . The early life environment and the epigenome. Biochim Biophys Acta 2009; 1790: 878–885.
Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet 2008; 82: 696–711.
Acknowledgements
Support was received from NIH grants MH-42088, MH-69056, MH-58922 and RR-25008. Dr Binder is supported by a Doris Duke Clinical Scientist Development Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Currently, Dr Nemeroff serves on the Scientific Advisory Board for the American Foundation for Suicide Prevention; AstraZeneca; NARSAD, PharmaNeuroboost and CeNeRx. He serves on the Board of Directors of American Foundation for Suicide Prevention; George West Mental Health Foundation; NovaDel Pharma, Mt Cook Pharma, Inc. He owns equity or is stock holder in Corcept; Revaax; NovaDel Pharma; CeNeRx, PharmaNeuroboost, Mt Cook Pharma. He is inventor on the following patents: method and devices for transdermal delivery of lithium (US 6,375,990 B1) and method to estimate serotonin and norepinephrine transporter occupancy after drug treatment using patient or animal serum (provisional filing April, 2001). Currently, Dr Binder receives grant support from NIMH and the Doris Duke charitable foundation.
Rights and permissions
About this article
Cite this article
Binder, E., Nemeroff, C. The CRF system, stress, depression and anxiety—insights from human genetic studies. Mol Psychiatry 15, 574–588 (2010). https://doi.org/10.1038/mp.2009.141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2009.141
Keywords
This article is cited by
-
CRF regulates pain sensation by enhancement of corticoaccumbal excitatory synaptic transmission
Molecular Psychiatry (2024)
-
Chia seeds oil ameliorate chronic immobilization stress-induced neurodisturbance in rat brains via activation of the antioxidant/anti-inflammatory/antiapoptotic signaling pathways
Scientific Reports (2023)
-
Relationship between adverse childhood experiences and anxiety symptoms among Chinese adolescents: The role of self-compassion and social support
Current Psychology (2023)
-
The independent association between salivary alpha-amylase activity and arterial stiffness in Japanese men and women: the Toon Health Study
Hypertension Research (2022)
-
Brain-wide perception of the emotional valence of light is regulated by distinct hypothalamic neurons
Molecular Psychiatry (2022)