Abstract
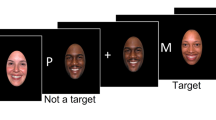
We combined functional imaging and genetics to investigate the behavioral and neural effects of a dysbindin-1 (DTNBP1) genotype associated with the expression level of this important synaptic protein, which has been implicated in schizophrenia. On a working memory (WM) task for emotional faces, participants with the genotype related to increased expression showed higher WM capacity for happy faces compared with the genotype related to lower expression. Activity in several task-related brain areas with known DTNBP1 expression was increased, including hippocampal, temporal and frontal cortex. Although these increases occurred across emotions, they were mostly observed in areas whose activity correlated with performance for happy faces. This suggests effects of variability in DTNBP1 on emotion-specific WM capacity and region-specific task-related brain activation in humans. Synaptic effects of DTNBP1 implicate that altered dopaminergic and/or glutamatergic neurotransmission may be related to the increased WM capacity. The combination of imaging and genetics thus allows us to bridge the gap between the cellular/molecular and systems/behavioral level and extend the cognitive neuroscience approach to a comprehensive biology of cognition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Blokland G, McMahon K, Hoffman J, Zhu G, Meredith M, Martin N et al. Quantifying the heritability of task-related brain activation and performance during the N-back working memory task: a twin fMRI study. Biol Psychol 2008; 79: 70–79.
Ando J, Ono Y, Wright MJ . Genetic structure of spatial and verbal working memory. Behav Genet 2001; 31: 615–624.
Hariri A, Drabant E, Weinberger D . Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry 2006; 59: 888–897.
Donohoe G, Morris D, Clarke S, McGhee K, Schwaiger S, Nangle J et al. Variance in neurocognitive performance is associated with dysbindin-1 in schizophrenia: a preliminary study. Neuropsychologia 2007; 45: 454–458.
Donohoe G, Morris D, De Sanctis P, Magno E, Montesi J, Garavan H et al. Early visual processing deficits in dysbindin-associated schizophrenia. Biol Psychiatry 2008; 63: 484–489.
Burdick K, Lencz T, Funke B, Finn C, Szeszko P, Kane J et al. Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet 2006; 15: 1563–1568.
Fallgatter A, Herrmann M, Hohoff C, Ehlis A, Jarczok T, Freitag C et al. DTNBP1 (dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology 2006; 31: 2002–2010.
Bray N, Preece A, Williams N, Moskvina V, Buckland P, Owen M et al. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Hum Mol Genet 2005; 14: 1947–1954.
Weickert C, Straub R, McClintock B, Matsumoto M, Hashimoto R, Hyde T et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry 2004; 61: 544–555.
Bray N, Buckland P, Owen M, O'Donovan M . Cis-acting variation in the expression of a high proportion of genes in human brain. Hum Genet 2003; 113: 149–153.
Mattick J, Makunin I . Non-coding RNA. Hum Mol Genet 2006; 15 (Spec No 1): R17–R29.
Luciano M, Miyajima F, Lind PA, Bates TC, Horan M, Harris SE et al. Variation in the dysbindin gene and normal cognitive function in three independent population samples. Genes Brain Behav 2009; 8: 218–227.
Talbot K, Eidem W, Tinsley C, Benson M, Thompson E, Smith R et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest 2004; 113: 1353–1363.
Weickert C, Rothmond D, Hyde T, Kleinman J, Straub R . Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res 2008; 98: 105–110.
Dégenètais E, Thierry A, Glowinski J, Gioanni Y . Synaptic influence of hippocampus on pyramidal cells of the rat prefrontal cortex: an in vivo intracellular recording study. Cereb Cortex 2003; 13: 782–792.
Wall P, Messier C . The hippocampal formation—orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behav Brain Res 2001; 127: 99–117.
Guo AY, Sun J, Riley BP, Thiselton DL, Kendler KS, Zhao Z . The dystrobrevin-binding protein 1 gene: features and networks. Mol Psychiatry 2009; 14: 18–29.
Talbot K, Cho D, Ong W, Benson M, Han L, Kazi H et al. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet 2006; 15: 3041–3054.
Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet 2008; 40: 827–834.
Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN et al. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry 2009; advance online publication, 23 June 2009; doi: 10.1038/mp.2009.58.
Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N et al. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet 2004; 13: 2699–2708.
Thiselton DL, Vladimirov VI, Kuo PH, McClay J, Wormley B, Fanous A et al. AKT1 is associated with schizophrenia across multiple symptom dimensions in the Irish study of high density schizophrenia families. Biol Psychiatry 2008; 63: 449–457.
Tan HY, Nicodemus KK, Chen Q, Li Z, Brooke JK, Honea R et al. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J Clin Invest 2008; 118: 2200–2208.
Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O'Brien EP et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet 2003; 35: 84–89.
Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY et al. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol 2008; 181: 791–801.
Jentsch JD, Trantham-Davidson H, Jairl C, Tinsley M, Cannon TD, Lavin A . Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology 2009; 34: 2601–2608.
Kubota K, Kumamoto N, Matsuzaki S, Hashimoto R, Hattori T, Okuda H et al. Dysbindin engages in c-Jun N-terminal kinase activity and cytoskeletal organization. Biochem Biophys Res Commun 2008; 379: 191–195.
Murotani T, Ishizuka T, Hattori S, Hashimoto R, Matsuzaki S, Yamatodani A . High dopamine turnover in the brains of Sandy mice. Neurosci Lett 2007; 421: 47–51.
Dolan RJ . Emotion, cognition, and behavior. Science 2002; 298: 1191–1194.
Gur R, Nimgaonkar V, Almasy L, Calkins M, Ragland J, Pogue-Geile M et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry 2007; 164: 813–819.
Langeslag SJ, Morgan HM, Jackson MC, Linden DE, Van Strien JW . Electrophysiological correlates of improved short-term memory for emotional faces. Neuropsychologia 2009; 47: 887–896.
Jackson MC, Wolf C, Johnston SJ, Raymond JE, Linden DE . Neural correlates of enhanced visual short-term memory for angry faces: an FMRI study. PLoS ONE 2008; 3: e3536.
Jackson MC, Wu CY, Linden DE, Raymond JE . Enhanced visual short-term memory for angry faces. J Exp Psychol Hum Percept Perform 2009; 35: 363–374.
Kumamoto N, Matsuzaki S, Inoue K, Hattori T, Shimizu S, Hashimoto R et al. Hyperactivation of midbrain dopaminergic system in schizophrenia could be attributed to the down-regulation of dysbindin. Biochem Biophys Res Commun 2006; 345: 904–909.
Iizuka Y, Sei Y, Weinberger D, Straub R . Evidence that the BLOC-1 protein dysbindin modulates dopamine D2 receptor internalization and signaling but not D1 internalization. J Neurosci 2007; 27: 12390–12395.
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC . Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33: 636–647.
Goebel R, Esposito F, Formisano E . Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp 2006; 27: 392–401.
Peelen MV, Downing PE . Within-subject reproducibility of category-specific visual activation with functional MRI. Hum Brain Mapp 2005; 25: 402–408.
Williams M, McGlone F, Abbott D, Mattingley J . Stimulus-driven and strategic neural responses to fearful and happy facial expressions in humans. Eur J Neurosci 2008; 27: 3074–3082.
Sambataro F, Dimalta S, Di Giorgio A, Taurisano P, Blasi G, Scarabino T et al. Preferential responses in amygdala and insula during presentation of facial contempt and disgust. Eur J Neurosci 2006; 24: 2355–2362.
Rissman J, Gazzaley A, D′Esposito M . Dynamic adjustments in prefrontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cereb Cortex 2008; 18: 1618–1629.
LoPresti M, Schon K, Tricarico M, Swisher J, Celone K, Stern C . Working memory for social cues recruits orbitofrontal cortex and amygdala: a functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. J Neurosci 2008; 28: 3718–3728.
Rolls E . The representation of information about faces in the temporal and frontal lobes. Neuropsychologia 2007; 45: 124–143.
Blasi G, Mattay V, Bertolino A, Elvevåg B, Callicott J, Das S et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci 2005; 25: 5038–5045.
Canli T, Omura K, Haas B, Fallgatter A, Constable R, Lesch K . Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci USA 2005; 102: 12224–12229.
Schott B, Seidenbecher C, Fenker D, Lauer C, Bunzeck N, Bernstein H et al. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci 2006; 26: 1407–1417.
Williams N, Preece A, Morris D, Spurlock G, Bray N, Stephens M et al. Identification in 2 independent samples of a novel schizophrenia risk haplotype of the dystrobrevin binding protein gene (DTNBP1). Arch Gen Psychiatry 2004; 61: 336–344.
Sachs G, Steger-Wuchse D, Kryspin-Exner I, Gur R, Katschnig H . Facial recognition deficits and cognition in schizophrenia. Schizophr Res 2004; 68: 27–35.
Tsoi D, Lee K, Khokhar W, Mir N, Swalli J, Gee K et al. Is facial emotion recognition impairment in schizophrenia identical for different emotions? A signal detection analysis. Schizophr Res 2008; 99: 263–269.
Roberts A, Tomic D, Parkinson C, Roeling T, Cutter D, Robbins T et al. Forebrain connectivity of the prefrontal cortex in the marmoset monkey (Callithrix jacchus): an anterograde and retrograde tract-tracing study. J Comp Neurol 2007; 502: 86–112.
Kalkman H . The role of the phosphatidylinositide 3-kinase-protein kinase B pathway in schizophrenia. Pharmacol Ther 2006; 110: 117–134.
Egan M, Kojima M, Callicott J, Goldberg T, Kolachana B, Bertolino A et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003; 112: 257–269.
Canli T, Lesch K . Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci 2007; 10: 1103–1109.
Butcher L, Davis O, Craig I, Plomin R . Genome-wide quantitative trait locus association scan of general cognitive ability using pooled DNA and 500K single nucleotide polymorphism microarrays. Genes Brain Behav 2008; 7: 435–446.
Tsankova N, Renthal W, Kumar A, Nestler E . Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci 2007; 8: 355–367.
Fish E, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M et al. Epigenetic programming of stress responses through variations in maternal care. Ann NY Acad Sci 2004; 1036: 167–180.
Klein M, Lioy D, Ma L, Impey S, Mandel G, Goodman R . Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci 2007; 10: 1513–1514.
Schratt G, Tuebing F, Nigh E, Kane C, Sabatini M, Kiebler M et al. A brain-specific microRNA regulates dendritic spine development. Nature 2006; 439: 283–289.
Wayman G, Davare M, Ando H, Fortin D, Varlamova O, Cheng H et al. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci USA 2008; 105: 9093–9098.
Acknowledgements
This work was supported by the Wellcome Trust, Grant no. 077185/05Z, the Wales Institute of Cognitive Neuroscience (WICN) and the North West Wales NHS Trust. We thank Tony Bedson and the radiography team at Ysbyty Gwynedd, Bangor for the acquisition of the imaging data, Tony Bedson and Stefanie Linden for taking of blood samples, Robert Walters, head of laboratory services at Ysbyty Gwynedd, for help with the blood sample logistics, Chris Whitaker for expert advice on statistics, John Parkinson for helpful comments on the paper and all our participants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Wolf, C., Jackson, M., Kissling, C. et al. Dysbindin-1 genotype effects on emotional working memory. Mol Psychiatry 16, 145–155 (2011). https://doi.org/10.1038/mp.2009.129
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2009.129
Keywords
This article is cited by
-
α-Dystrobrevin knockout mice have increased motivation for appetitive reward and altered brain cannabinoid receptor 1 expression
Acta Neuropathologica Communications (2022)
-
Cortical copper transporter expression in schizophrenia: interactions of risk gene dysbindin-1
Journal of Neural Transmission (2021)
-
Loss of dysbindin-1 affects GABAergic transmission in the PFC
Psychopharmacology (2019)
-
Variations in Dysbindin-1 are associated with cognitive response to antipsychotic drug treatment
Nature Communications (2018)
-
Dysregulation of Specialized Delay/Interference-Dependent Working Memory Following Loss of Dysbindin-1A in Schizophrenia-Related Phenotypes
Neuropsychopharmacology (2017)