Abstract
Thyroid tumors formerly classified as non-invasive encapsulated follicular variant of papillary thyroid carcinoma were recently renamed ‘non-invasive follicular thyroid neoplasm with papillary-like nuclear features’. The current study investigated the frequency of lymph node metastasis and mutational profile of encapsulated follicular variant in the setting of a clinical practice where central neck dissection was the standard of practice. We defined the impact of rigid diagnostic criteria by regrouping such tumors based on the complete absence of papillae or presence of ≤1% papillae. Of a total of 6,269 papillary thyroid carcinomas, 152 tumors fulfilled the criteria for encapsulated follicular variant. The results were stratified according to two different diagnostic cutoff criteria with respect to the extent of papillae. When the cutoff of 1% papillae was used, the rates of lymph node metastasis and BRAFV600E mutation were 3% and 10% in non-invasive tumors and 9% and 4% in invasive tumors, respectively. Despite the lack of invasive growth, one patient with BRAFV600E mutant-tumor displaying predominant follicular growth and subtle papillae developed a bone metastasis. When absence of papillary structure was applied as rigid diagnostic criteria, no BRAFV600E mutation was found in all tumors. However, central lymph node micrometastasis still occurred in 3% of non-invasive tumors. Non-V600E BRAF and RAS mutations were detected in 4% and 47% of non-invasive tumors, respectively. Our findings suggest that non-invasive follicular thyroid neoplasm with papillary-like nuclear features should not be regarded as a benign thyroid neoplasm as it can present with lymph node micrometastasis and should not be diagnosed in the presence of even a single papillary structure. Our findings underscore the original American Thyroid Association recommendation that defined non-invasive encapsulated follicular variants as low risk thyroid cancers. Clinical surveillance similar to low risk differentiated thyroid cancers and capture of this diagnostic category by Cancer Registries should be considered.
Similar content being viewed by others
Main
The worldwide incidence of thyroid cancer is on the rapid rise.1, 2, 3, 4, 5 The increased incidence has been linked to over detection of small papillary thyroid carcinomas due to increased sonographic screening in asymptomatic healthy populations.1, 2, 3, 4, 5, 6 In addition, the increased recognition of encapsulated follicular variant of papillary thyroid carcinoma has been thought to be a contributing factor for the increasing incidence.7, 8, 9 Recent studies have demonstrated that non-invasive encapsulated follicular variant of papillary thyroid carcinoma accounts for around 10–20% of all thyroid cancers diagnosed in some practices in North America and Europe.9, 10 In fact, the diagnosis of non-invasive encapsulated follicular variant of papillary thyroid carcinoma has been a challenge given the lack of universal agreement on the low threshold diagnostic nuclear alterations of papillary thyroid carcinoma.11, 12 As a consequence, a significance intra- and inter-observer variation has been reported even among experts in the distinction of papillary thyroid carcinoma.13, 14 Despite controversies in the field, it has been shown that non-invasive encapsulated follicular variant of papillary thyroid carcinoma overall follows a less aggressive clinical course than infiltrative follicular variant of papillary thyroid carcinoma and other variants of papillary thyroid carcinoma.15, 16 Consistent with this approach, several social health systems have developed practice guidelines to adopt a conservative approach for non-invasive encapsulated follicular variant of papillary thyroid carcinomas. However, a conservative approach has not been undertaken globally. In response, a group of experts recently suggested that non-invasive encapsulated follicular variant of papillary thyroid carcinomas be re-classified as ‘non-invasive follicular thyroid neoplasm with papillary-like nuclear features’ instead of keeping the word ‘cancer’ in the nomenclature.10, 11
The precise frequency of lymph node metastasis in low risk thyroid cancer and especially in non-invasive follicular thyroid neoplasm with papillary-like nuclear features is difficult to determine reliably in most clinical series as almost all specimens lack routine neck dissection.10, 17 Unlike most Western practices, surgical practice in South Korea has generally adopted prophylactic central lymph node dissection in thyroid cancer surgery.18 The strength of such a surgical practice is that it provides invaluable insights on the status of lymph node status in different forms of encapsulated follicular variant of papillary thyroid carcinomas including non-invasive lesions that would be classified as non-invasive follicular thyroid neoplasm with papillary-like nuclear features.
At the molecular level, it has been clear that encapsulated follicular variant of papillary thyroid carcinoma, follicular adenoma, and follicular carcinoma share similar molecular alterations characterized by a high frequency of RAS mutations, the presence of PAX8-PPARγ rearrangement, and rare BRAF mutations.10, 19, 20, 21, 22, 23, 24 Absence of BRAFV600E is regarded as a characteristic feature of non-invasive encapsulated follicular variant of papillary thyroid carcinoma23, 24 but BRAFV600E has been found in invasive forms of encapsulated follicular variant of papillary thyroid carcinoma with varying rates from 0 to 30.9%.20, 22, 25, 26, 27, 28 One of the possible contributing factors to the variable prevalence of BRAFV600E is different pathologic diagnostic criteria used in the studies to select encapsulated follicular variant of papillary thyroid carcinomas. While some experts have recommended the absence of papillae as a criterion for the distinction of follicular variant of papillary thyroid carcinoma from classical papillary thyroid carcinoma with predominant follicular growth,29 an arbitrary cutoff of 1% papillary growth was allowed in the non-invasive follicular thyroid neoplasm with papillary-like nuclear features consensus study.10
In this study, we aimed to investigate the frequency of lymph node metastasis and mutational profile in completely examined thyroidectomy specimens with non-invasive and invasive encapsulated follicular variant of papillary thyroid carcinomas in the setting of a surgical practice where the routine central neck dissection was the standard of practice. In addition, we examined the impact of rigid diagnostic criteria by regrouping such tumors based on the absence of any papillae or presence of ≤1% papillae.
Materials and methods
Study Cohort and Sample Collection
This study evaluated patients who underwent thyroidectomy at Seoul St Mary’s Hospital from January 2008 to December 2014. Of a total of 6,269 patients diagnosed with papillary thyroid carcinoma, 175 patients had tumors that fulfilled the published criteria for encapsulated follicular variant of papillary thyroid carcinoma (Figure 1). The tumor capsule and tumor/adjacent thyroid tissue interface along with the remaining tumor content was submitted entirely for microscopic examination. In addition, the histological examination of the entire thyroid gland was also performed. All histologic slides were examined independently by two experienced pathologists (UC and CKJ) with a special interest in thyroid pathology and blinded to the clinical and molecular results. Serial and deeper sections were performed whenever required. Tumors with equivocal histologic features (e.g., rare scattered papillary structures, growth pattern, and questionable capsular or vascular invasion), lymph node metastasis, or BRAFV600E mutation were also reviewed by an external expert in thyroid pathology (OM).
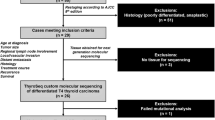
The selection of encapsulated follicular variant of papillary thyroid carcinoma was made according to the consensus diagnostic criteria proposed in the paper ‘Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma’.10 The major diagnostic criteria for the encapsulated follicular variant of papillary thyroid carcinoma were as follows (Figure 2): (1) complete or partial encapsulation or clear demarcation of the tumor from adjacent non-tumor tissue, (2) lack of invasion, (3) follicular growth pattern, (4) nuclear features of papillary thyroid carcinoma (enlargement, crowding/overlapping, elongation, irregular contours, grooves, pseudoinclusions and chromatin clearing).10 Exclusion criteria were as follows (Figure 2): (1) when entire tumor tissue was not submitted for microscopic examination, (2) cases with a second tumor of >1 cm, (3) tumors with any of the following: any amount of dedifferentiated component, >1% papillary structure, necrosis, psammoma bodies, mitotic figures ≥3 per 10 high-power fields (× 400), or any special cytomorphologic variant of papillary thyroid carcinoma according to the consensus study of encapsulated follicular variant of papillary thyroid carcinoma.10 Encapsulated follicular variant of papillary thyroid carcinomas were subdivided into invasive encapsulated follicular variant of papillary thyroid carcinoma and non-invasive encapsulated follicular variant of papillary thyroid carcinoma according to the status of tumor capsular and vascular invasion. The presence of vascular invasion was acknowledged when invasion foci were present within or beyond the capsule, and when invasive tumor cells protruded into the lumen of the vessel were covered by endothelial cells or attached to the vessel wall or associated with thrombus formation. As a result, 23 cases were excluded due to unmet inclusion criteria and 152 patients with encapsulated follicular variant of papillary thyroid carcinoma were enrolled in this study when the diagnostic cutoff of ≤1% papillae is applied (Figure 1).
Morphologic features of non-invasive (a–c) and invasive (d–f) encapsulated follicular variant of papillary thyroid carcinoma. (a) A non-invasive tumor illustrated in this photograph. The tumor was clearly demarcated from the surrounding non-lesional thyroid parenchyma by a thin capsule. Serial sections of the tumor and the thyroid tissue were entirely submitted for the microscopic examination. (b) The grossly illustrated tumor was completely composed of follicular structures surrounded by a fibrous capsule (× 40). (c) The tumor cells displayed nuclear features of papillary thyroid carcinoma characterized by nuclear enlargement, crowding/overlapping, irregular contours, grooves and chromatin clearing (× 400). (d) This photomicrograph illustrates an encapsulated follicular variant of papillary thyroid carcinoma with a focus of invasive growth (arrow) (× 12.5). (e) A high magnification from the site of invasive growth is illustrated (× 100). (f) This tumor displayed nuclear features of papillary thyroid carcinoma characterized by nuclear membrane irregularities in enlarged nuclei (× 400).
Following the ethical board approval of Seoul St Mary’s Hospital, The Catholic University of Korea (KC16RISI0038), patient demographics data and follow-up information were retrospectively collected from the hospital medical records as well as the thyroid tumor registry. Pathologic stages were categorized according to the 7th edition of the TNM classification and stage grouping by the American Joint Committee on Cancer.
Molecular Analysis of BRAF, NRAS, HRAS, and KRAS Genes
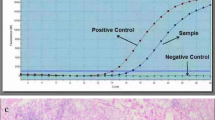
Genomic DNA was extracted from two 10-μm sections of formalin-fixed, paraffin-embedded tissue blocks. The representative slides from invasive and non-invasive tumors were selected and marked. Subsequently, the neoplastic tissue was manually microdissected under a stereomicroscope. DNA extraction was performed using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s instruction. Exon 15 of BRAF gene, exon 3 of NRAS and HRAS genes, and exons 2 and 3 of KRAS gene were amplified using polymerase chain reaction (PCR) with following primers; BRAF exon 15–224 bp-forward (5′-TCATAATGCTTGCTCTGATAGGA-3′) and reverse (5′-GGCCAAAAATTTAATCAGTGGA-3′); NRAS-exon 3-197 bp-forward (5′-CCCCTTACCCTCCACACC-3′) and reverse (5′-GAGGTTAATATCCGCAAATGACTT-3′); HRAS-exon 3-201 bp-forward (5′-GTCCTCCTGCAGGATTCCTA-3′) and reverse (5′-CGGGGTTCACCTGTACT-3′); KRAS-exon 2-263 bp-forward (5′-GGTGAGTTTGTATTAAAAGGTACTGG-3′) and reverse (5′-TCCTGCACCAGTAATATGCA-3′); KRAS-exon 3-197 bp-forward (5′-GGTGCACTGTAATAATCCAGAC-3′) and reverse (5′- TGATTTAGTATTATTTATGGC-3′). The PCRconditions were as follows: an initial activation at 94 °C for 15 min; 35 cycles at 94 °C for 30 s, 51–57 °C for 30 s and 72 °C for 30 s; final extension at 72 °C for 10 min. The amplicons were evaluated by 2% agarose gel electrophoresis and purified through QIAquick PCR purification kit (Qiagen). Sequencing of the amplified PCR products were done by Sanger sequencing using the same PCR primers.20, 30, 31 For tumors with rare BRAF mutations (mutations other than BRAFV600E), (1) re-amplification, (2) repeat DNA sequencing, and (3) bidirectional sequencing using two new sets of primers were performed to confirm the authenticity, as described by us earlier.32 Moreover, to identify the nucleotide composition of deletion mutations, PCR amplicons were cloned using the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA, USA). The descriptions of the mutations followed the ‘Guidelines for mutation nomenclature’ from Human Genome Variation Society.33 For BRAF gene analysis, NBCI reference sequences-NG_007873.1 and NM_004333.4 were used (www.ncbi.nlm.nih.gov/nuccore).
Statistical Analysis
To analyze the correlation between mutational status and clinicopathologic parameters, χ2-test or Fisher's exact test was used when appropriate. Analyses were done by using SPSS 21.0 (IBM, Armonk, NY, USA).
Results
The results were stratified into two groups based on the extent of papillae in the tumor (Figure 1). When the cutoff of ≤1% papillary growth was used, 152 tumors were classified as encapsulated follicular variant of papillary thyroid carcinoma (105 non-invasive and 47 invasive encapsulated follicular variant of papillary thyroid carcinomas). When the cutoff of 0% papillae (strict diagnostic criteria) was applied, there were 95 non-invasive and 45 invasive encapsulated follicular variant of papillary thyroid carcinomas.
Clinicopathologic and Molecular Features of Non-Invasive and Invasive Encapsulated Follicular Variant of Papillary Thyroid Carcinomas Classified by the Inclusion of ≤1% Papillary Structure
Table 1 summarizes clinicopathologic characteristics on 152 tumors classified as encapsulated follicular variant of papillary thyroid carcinoma when the cutoff of 1% papillary structure was applied. In this group, there were no significant differences in the baseline characteristics between invasive and non-invasive tumors. Synchronous papillary microcarcinoma was identified in 19 (18%) of 105 non-invasive encapsulated follicular variant of papillary thyroid carcinomas and 11 (23%) of 47 invasive encapsulated follicular variant of papillary thyroid carcinomas. The median follow-up was 37 months (range 17–96 months).
Since 20 tumors were incidentally discovered, lymph node dissection was available from 132 of 152 patients in this group. Lymph node metastasis was identified in 3 (3%) of 88 non-invasive encapsulated follicular variant of papillary thyroid carcinomas and 4 (9%) of 44 invasive encapsulated follicular variant of papillary thyroid carcinomas with lymph nodes tissues available for evaluation. In 7 cases with lymph node metastasis, the histology of metastatic disease showed micrometastasis (< 2 mm by definition), entirely of follicular growth pattern and nuclear features of papillary thyroid carcinoma similar to the main tumor (Figure 3). Lateral lymph node metastasis was only found in one patient with invasive encapsulated follicular variant of papillary thyroid carcinoma.
Non-invasive encapsulated follicular variant papillary thyroid carcinoma (currently known as non-invasive follicular thyroid neoplasm with papillary-like nuclear features) presenting with central node micrometastasis. No additional thyroid malignancy was seen in the entirely submitted thyroidectomy specimen. (a) Low power view of case 1 showing non-invasive follicular thyroid neoplasm with papillary-like nuclear features composed completely of follicular growth lacking any papillae (× 40). The inserted photomicrograph illustrates follicular epithelial cells with nuclear alterations of papillary thyroid carcinoma (× 400). (b) A representative section from a central lymph node showing micrometastatic tumor deposit (× 40). The inserted photomicrograph illustrates nuclear alterations of papillary thyroid carcinoma (× 400). (c) The second case of non-invasive follicular thyroid neoplasm with papillary-like nuclear features with central micrometastatic lymph node disease. The tumor displayed mixed microfollicular and macrofollicular growth pattern (× 40). (d) The tumor cells showed nuclear alterations of papillary thyroid carcinoma as well as ‘sprinkling sign’ (× 400). (e) A representative section from a central neck lymph node showing micrometastatic tumor deposit measuring less than 2 mm (× 40). (f) The micrometastatic tumor cells displayed enlarged nuclei and chromatin clearing. These cells retained their follicular arrangement in the metastatic lesion (× 400).
The molecular studies for BRAF alterations were available for 152 tumors in this group. The overall BRAF mutation rate was 13% (14/105) in the non-invasive encapsulated follicular variant of papillary thyroid carcinoma and 9% (4/47) in the invasive encapsulated follicular variant of papillary thyroid carcinoma (Table 2). The BRAF alterations in the 105 non-invasive group included c.1799T>A (BRAFV600E) (n=10), c.1801A>G (BRAFK601E) (n=2), c.1799_1801del (BRAF V600E_K601delinsE) (n=1), and c.1803_1814del (BRAF V601E_S605delinsN) (n=1). The BRAF alterations in the 47 invasive tumors included BRAFV600E (n=2) and BRAFK601E (n=2). The rate of BRAFV600Emutation status did not yield any statistical difference between non-invasive and invasive tumors when the arbitrary 1% cutoff was applied (P=0.344).
To our knowledge, the BRAF V601E_S605delinsN has not been reported in human cancers. This type of mutation was detected in a 45 year old female with a 1.3 cm non-invasive encapsulated follicular variant of papillary thyroid carcinoma. The postoperative stage was pT1bN0M0. Cloning and sequencing analysis showed 12-nucleotide deletion between positions c.1803 and c.1814 (c.1803_1814del; Figure 4). This mutation leads to the deletion of 5 amino acids, Lys-Ser-Ala-Trp-Ser between codons 600 and 606, and substitution of asparagine in that position (p.Lys601_Ser605delinsAsn).
Clinicopathologic Features of Tumors with BRAFV600E
The BRAFV600E mutation was found in 12 (8%) of 152 encapsulated follicular variant of papillary thyroid carcinomas and was more frequently detected in males (P=0.02). However, the BRAFV600Estatus did not yield a statistical significance with respect to the tumor size, age, extent of surgery, synchronous papillary microcarcinoma, tumor invasiveness, lymph node metastasis, distant metastasis, and tumor stage (Table 3).
Histologically, all 12 encapsulated follicular variant of papillary thyroid carcinomas (10 non-invasive and 2 invasive encapsulated follicular variant of papillary thyroid carcinoma) harboring BRAFV600E mutation displayed rare or scattered true papillary structures which were present in less than 1% of the tumor volume (Figure 5). No papillae were detected in encapsulated follicular variant of papillary thyroid carcinomas with BRAF alterations other than BRAFV600E mutations. There was no difference between the BRAFV600E mutation status and histologic features including nuclear features, stromal fibrosis, and thickness of fibrous capsule.
BRAFV600E harboring papillary carcinoma with scattered papillae (a, b—case no. 1; c, d—case no. 2; e, f—case no. 3). Only a few sections from such tumors displayed scattered papillae in the background of predominant follicular growth accounting for at least 99% of the tumor volume (arrows indicated papillary structures). Papillary structures contained fibrovascular stroma (arrows).
Of 12 tumors with BRAFV600E, one developed a distant metastasis at the time of initial presentation. The patient was a 53 year old male who presented with right hip pain due to a cystic bone lesion at the right iliac bone. Incisional biopsy of the iliac bone revealed a metastatic well differentiated thyroid cancer. The patient underwent total thyroidectomy with central lymph node dissection and wide excision of the metastatic bone lesion. The thyroidectomy specimen was submitted in toto for microscopic examination. The pathologic examination of the thyroidectomy specimen showed a 0.6 cm non-invasive papillary thyroid carcinoma with predominant follicular growth and scattered papillary structures (in up to 1%) in the right thyroid lobe. The metastatic right iliac tumor measured 4.7 cm and displayed a similar cytomorphology (Figure 6). Despite serial and deeper sections, there was no evidence of vascular or capsular invasion. No mitosis or necrosis was also noted. No metastasis was found in the cervical lymph nodes. Both primary and metastatic tumors showed BRAFV600E mutation. The patient had radioactive iodine treatment with a total cumulative dose of 550 mCi. There was no structural disease recurrence during a follow-up of 86 months.
Tumor harboring BRAFV600E and classified as non-invasive encapsulated follicular variant papillary thyroid carcinoma presented with a bone metastasis. (a) The thyroidectomy specimen was submitted in toto and only a single focus of malignancy was noted. Serial and deeper sections from this tumor lacked evidence of invasive growth (× 40). (b) A single focus of papillary structure was noted (× 200). (c) The tumor displayed predominantly a microfollicular growth as well as nuclear features of papillary thyroid carcinoma (× 400). (d) Right pelvic metastasis (arrows) was highlighted on bone scan and positron emission tomography–computed tomography. Pelvic computed tomography showed an osteolytic metastatic tumor of the bone. (e) Gross examination of the resected bone identified the presence of a mixed cystic and solid mass centered in the medulla. (f) Light microscopic examination from the metastatic right pelvic mass revealed a papillary thyroid carcinoma with predominant follicular growth admixed with scattered papillae (× 100).
Clinicopathologic Characteristics and Molecular Features of Non-Invasive and Invasive Encapsulated Follicular Variant of Papillary Thyroid Carcinoma Classified by Using Rigid Diagnostic Criteria (No Papillary Structure)
In this group, papillary thyroid carcinomas showing scattered true papillary structures (≤1% papillae) in the background of predominant follicular growth were re-classified as classical papillary thyroid carcinoma with predominant follicular growth and excluded from the encapsulated follicular variant of papillary thyroid carcinoma cohort. Accordingly, the number of patients with the diagnostic category of non-invasive encapsulated follicular variant of papillary thyroid carcinoma (currently would have been re-classified as non-invasive follicular thyroid neoplasm with papillary-like nuclear features) dropped from 105 to 95 by using rigid diagnostic criteria. Demographics and clinicopathologic features of 95 patients with non-invasive follicular thyroid neoplasm with papillary-like nuclear features are shown in Table 4. There was a female predominance (female to male ratio=2.1:1) and the median age was 47 years.
Since 20 tumors were incidentally discovered, lymph node dissection was available only from 120 of 140 patients in this group. Two patients (3%) with non-invasive follicular thyroid neoplasm with papillary-like nuclear features (unassociated with synchronous papillary microcarcinoma or other forms of thyroid cancer) had micrometastatic lymph nodes in the central neck compartment (Figure 3). The number of metastatic lymph nodes was 3 and 1, respectively. The micrometastatic foci were considered for molecular subtyping; however, they disappeared in the deeper sections. BRAF gene mutations other than BRAFV600E were detected in 4 (4%) of 95 non-invasive follicular thyroid neoplasms with papillary-like nuclear features. These alterations included BRAFK601E (n=2), BRAF V600E_K601delinsE (n=1), and BRAF V601E_S605delinsN (n=1) as above described.
RAS mutational analysis was available in 127 cases (38 invasive encapsulated follicular variant of papillary thyroid carcinomas and 89 non-invasive follicular thyroid neoplasms with papillary-like nuclear features) of this group. Overall mutation frequency of three RAS genes was 47% (60/127). NRAS, HRAS and KRAS mutation rates were 34, 9 and 4%, respectively (Table 5). RAS and BRAF mutations were mutually exclusive. There was no significant correlation between RAS mutational status and clinicopathologic features (tumor invasiveness, tumor size, age, extent of surgery, synchronous microcarcinoma, lymph node metastasis, distant metastasis, and tumor stage).
The number of invasive encapsulated follicular variant of papillary thyroid carcinoma dropped from 47 to 45 when the cutoff of 0% papillae was applied. The excluded two invasive tumors displayed scattered papillae and harbored BRAFV600E. Basic characteristics of patients with invasive encapsulated follicular variant of papillary thyroid carcinomas lacking any papillae are shown in Table 4. Lymph node metastasis was found in 3 (7%) of 42 invasive encapsulated follicular variant of papillary thyroid carcinomas with available central neck dissection. One invasive encapsulated follicular variant of papillary thyroid carcinoma had two metastatic lesions in lateral lymph nodes.
There was no significant difference between the non-invasive encapsulated follicular variant of papillary thyroid carcinomas (non-invasive follicular thyroid neoplasm with papillary-like nuclear features) and invasive encapsulated follicular variant of papillary thyroid carcinoma with respect to the tumor size, age, gender, presence of synchronous microcarcinoma, rate of lymph node metastasis, tumor stage, and mutational status of BRAF and RAS genes (Table 4). No biochemical or structural recurrence occurred in both non-invasive encapsulated follicular variant of papillary thyroid carcinoma (non-invasive follicular thyroid neoplasm with papillary-like nuclear features) and invasive encapsulated follicular variant of papillary thyroid carcinoma patients during the follow-up period (median 36, range 17–96 months).
Discussion
The diagnostic category of encapsulated follicular variant of papillary thyroid carcinoma refers to follicular variant of papillary thyroid carcinomas that shows either a well-defined complete capsule or clear demarcation without any form of invasive growth.29 There has been a considerable discrepancy among pathologists regarding the diagnosis of encapsulated follicular variant of papillary thyroid carcinoma because tumors with follicular growth pattern can show focal or subtle nuclear features of papillary thyroid carcinoma. The recently published diagnostic criteria sought to provide a diagnostic standard for invasive encapsulated follicular variant of papillary thyroid carcinoma and non-invasive follicular thyroid neoplasm with papillary-like nuclear features (formerly known as non-invasive encapsulated follicular variant of papillary thyroid carcinoma).10 This initiative also introduced a molecular based nuclear scoring system that resulted in a high inter-observer reproducibility for diagnosing nuclear alterations among the working group expert pathologists.10
In our non-invasive follicular thyroid neoplasm with papillary-like nuclear features case series, mutation rates were 47% for three RAS genes and 4% for non-V600E mutations of BRAF gene. These findings are in alignment with the results of a previous study in which RAS and BRAFK601E mutations were found in 30 and 4% of non-invasive follicular thyroid neoplasms with papillary-like nuclear features (n=27), respectively.10
Earlier studies showed that BRAFV600E mutation could be detected in infiltrative encapsulated follicular variant of papillary thyroid carcinoma, but not in non-invasive encapsulated follicular variant of papillary thyroid carcinoma (currently known as non-invasive follicular thyroid neoplasm with papillary-like nuclear features).10, 24, 28 In fact, we thought that several factors could contribute to the detection of BRAFV600E in tumors with predominant follicular growth. Among these possibilities, the use of less rigid morphological criteria to distinguish classical variant papillary thyroid carcinomas from follicular variant of papillary thyroid carcinomas should be questioned. In addition, subcentimeter tumors (papillary microcarcinoma) in which scattered papillary structures may be discerned could have been inaptly included as encapsulated follicular variant of papillary thyroid carcinoma.28 Despite the lack of mutual agreement among experts, an arbitrary cutoff of 1% papillary growth was introduced in the non-invasive follicular thyroid neoplasm with papillary-like nuclear features consensus paper.10 In the current study, BRAFV600E mutation was detected in 12 (8%) of 152 encapsulated follicular variant of papillary thyroid carcinomas when by using the arbitrary cutoff of 1% papillae. However, our retrospective review of both non-invasive and invasive tumors harboring BRAFV600E mutation identified scattered true papillary structure accounting for less than 1% of the tumor volume in such cases. Our results justified that the proposed arbitrary cutoff of 1% papillae may give rise to diagnostic discrepancies and can lead to a misclassification of biologically more aggressive variants of papillary thyroid carcinomas as non-invasive follicular thyroid neoplasm with papillary-like nuclear features. The most dramatic example was seen in a BRAFV600E harboring tumor with predominant follicular growth tumor that presented with distant metastasis. In fact, this tumor was re-classified as a classical variant papillary thyroid carcinoma with predominant follicular growth. The findings of this cohort suggest that the diagnosis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive encapsulated follicular variant of papillary thyroid carcinoma should only be restricted to follicular patterned tumors lacking any papillary structure. Therefore, tumors with scattered papillae (even in the presence of a single true papillary structure) should be considered as a classical variant papillary thyroid carcinoma as suggested by other experts.29
At this time, non-invasive encapsulated follicular variant of papillary thyroid carcinoma or non-invasive follicular thyroid neoplasm with papillary-like nuclear features remains a surgical diagnostic category and it does not warrant an aggressive treatment with total thyroidectomy and radioactive iodine. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features demonstrates molecular phenotypes similar to those of follicular patterned neoplasms including follicular adenoma, follicular carcinoma, and infiltrative encapsulated follicular variant of papillary thyroid carcinoma.10 Based on the literature review, the mean rates of lymph node metastasis have been reported to occur in 12, 21, and 40% of infiltrative encapsulated follicular variant of papillary thyroid carcinomas, follicular variant of papillary thyroid carcinomas (regardless of encapsulation and invasion status), and classical papillary thyroid carcinomas, respectively.17, 34 No lymph node metastasis has been reported in recent studies of non-invasive follicular thyroid neoplasms with papillary-like nuclear features.10, 17 However, the latter should be interpreted with caution as most surgical practices have not adopted a routine prophylactic lymph node dissection in the absence of clinical lymph node involvement, thus, the status of pN (pathological node status) is mostly unavailable in the vast majority of cases falling into the category of non-invasive follicular thyroid neoplasm with papillary-like nuclear features.
Unlike most Western practices, surgical practice in South Korea has generally adopted prophylactic central lymph node dissection in thyroid cancer surgery.18 The strength of such a surgical practice is that it provides invaluable insights on the status of lymph node status in different forms of encapsulated follicular variant of papillary thyroid carcinomas including non-invasive lesions that would be classified as non-invasive follicular thyroid neoplasm with papillary-like nuclear features. In the current series, the rate of metastatic nodal disease was 3% (2/78) in non-invasive follicular thyroid neoplasm with papillary-like nuclear features and 7% (3/42) in invasive encapsulated follicular variant of papillary thyroid carcinoma when the tumor was classified in the absence of papillae. The overall rates of lymph node metastasis were higher when the diagnostic categories of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive encapsulated follicular variant of papillary thyroid carcinoma were rendered in the setting of ≤1% papillae.
Patients with non-invasive follicular thyroid neoplasm with papillary-like nuclear features (based on 0% papillae) have not developed loco-regional or distant recurrence in the published series, and our cohorts identified only micrometastasis in central neck lymph nodes.10, 17 The detection of such microscopic deposits would be impossible if central neck dissection was not performed. However, the overall findings are still in line with the categorization of non-invasive follicular thyroid neoplasm with papillary-like nuclear features as a form of low risk papillary carcinoma (generally thought to have 1–2% risk of structural disease recurrence after initial therapy) as defined by the recurrence risk stratification system proposed by the most recent American Thyroid Association guidelines.35
An additional strength of the current study is related to the fact the entire tumor tissue was subjected to microscopic examination along with serial and/or deeper section. Despite its importance, the complete sampling of the tumor capsule interface is still not the standard of practice in most laboratories. While meticulous histological examination of the tumor capsule interface is the most important approach to exclude tumor capsular or vascular invasion, the authors believe that a careful examination of the entire tumor tissue is also critical to determine whether there are any scattered papillae, mitotic figures (≥3/10 HPF), necrosis, psammoma bodies, and other growth patterns. These observations also justify the need for evidence-based professional global practice guidelines on adequate tumor tissue sampling and validation of criteria used for invasive growth.
Another significant finding of this study is related to relatively low incidence of non-invasive follicular thyroid neoplasm with papillary-like nuclear features when submitting the entire tumor capsule and performing serial/deeper sectioning. The incidence of non-invasive follicular thyroid neoplasm with papillary-like nuclear features was estimated to be around 18% of total papillary thyroid carcinomas in the North America and Europe.9, 10, 25 In the current series, the rate of non-invasive encapsulated follicular variant of papillary thyroid carcinoma (non-invasive follicular thyroid neoplasm with papillary-like nuclear features) was 2% (105/6,269). While follicular variant of papillary thyroid carcinoma is still the second most common variant of papillary thyroid carcinoma in Korean population, the proportion of follicular variant of papillary thyroid carcinoma is relatively lower in Korea than North America and Europe.30, 36 Geographical and ethnic differences as well as non-unified diagnostic criteria could have influenced on this gap. However, one can also speculate that the incidence of non-invasive follicular thyroid neoplasm with papillary-like nuclear features might be overestimated in certain practices when the tumor tissue was not entirely submitted. Now that the consensus diagnostic criteria and the non-invasive follicular thyroid neoplasm with papillary-like nuclear features nomenclature are being adopted by the upcoming fourth edition of the WHO classification, additional epidemiology data will be gathered from different practices.
Additional findings related to molecular correlates of these neoplasms, the authors noted four encapsulated follicular variant of papillary thyroid carcinomas harboring BRAFK601E. This finding is in alignment with previous reports suggesting the association of K601E mutation with encapsulated follicular variant of papillary thyroid carcinoma rather than classic papillary thyroid carcinomas.27, 32, 37 Moreover, the authors identified a new BRAF mutation (c.1803_1814del in exon 15) that has not been reported before in thyroid cancer. This mutation results in the deletion of five amino acids, Lys-Ser-Ala-Trp-Ser positioned from codon 601 to 605, and subsequent substitution of asparagine in that position. The change of amino acids in this position is expected to have similar effect with that from BRAFK601E because the mutation occurs at the activation loop (A-loop binding pocket, residues 593-622) of the BRAF protein.27, 32 Oncogenic mutations in this location results in constitutive activation of the MAPK pathway. Protein alterations due to three uncommon mutations (c.1801A>G, c.1799_1801del, c.1803_1814del) were predicted using a computational analysis tool (Protein Variation Effect Analyzer), and all were predicted to have deleterious effect. However, exact mechanism on how c.1803_1814del affects protein function and its kinase activity require further clarification.
Conclusion
In contrast to Western literature, the rate of non-invasive follicular thyroid neoplasm with papillary-like nuclear features is significantly lower in Korea with a rate of 2%. This may be due to complete assessment of the tumor tissue; however, the possibility of an ethnical or environmental factor cannot also be excluded. More importantly, our results showed that the arbitrary cutoff of 1% misclassified non-invasive classical papillary thyroid carcinomas with predominant follicular architecture as non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Using the more rigid histologic criteria, all BRAFV600E-harboring encapsulated follicular variant of papillary thyroid carcinomas were re-classified as classic papillary thyroid carcinoma with predominant follicular growth as proposed by other experts.29 Therefore, the diagnosis of non-invasive follicular variant of papillary thyroid carcinoma (non-invasive follicular thyroid neoplasm with papillary-like nuclear features) and infiltrative follicular variant of papillary thyroid carcinoma should only be restricted to tumors displaying exclusive follicular pattern lacking true papillae. In addition, the adoption of routine central neck dissection even in the absence of clinical nodal disease showed that around 3% of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (completely submitted, assessed with serial sections, and unassociated with another focus of malignancy) presented with micrometastatic disease in the central neck lymph nodes. Despite the identification of micrometastasis, the follow-up data justified non-invasive follicular thyroid neoplasm with papillary-like nuclear features or non-invasive encapsulated follicular variant of papillary thyroid carcinoma being associated with an indolent clinical behavior identical to low risk papillary thyroid carcinomas. Therefore, no aggressive treatment with completion thyroidectomy and radioactive iodine is required for this diagnostic category. The concept of non-invasive follicular thyroid neoplasm with papillary-like nuclear features is still under evolution; however, labeling such tumors as benign diagnostic entities38 does not seem to be appropriate as conservative follow-up algorithms similar to other low risk thyroid cancers should be considered for non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Furthermore, these neoplasms still need to be captured by the Cancer Registries and very long term follow-up data from very large-scale population based studies is needed.
References
Davies L, Welch HG . Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164–2167.
Kent WD, Hall SF, Isotalo PA et al. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ 2007;177:1357–1361.
Colonna M, Guizard AV, Schvartz C et al. A time trend analysis of papillary and follicular cancers as a function of tumour size: a study of data from six cancer registries in France (1983-2000). Eur J Cancer 2007;43:891–900.
Farahati J, Geling M, Mader U et al. Changing trends of incidence and prognosis of thyroid carcinoma in lower Franconia, Germany, from 1981-1995. Thyroid 2004;14:141–147.
Harach HR, Escalante DA, Day ES . Thyroid cancer and thyroiditis in Salta, Argentina: a 40-yr study in relation to iodine prophylaxis. Endocr Pathol 2002;13:175–181.
Ahn HS, Kim HJ, Welch HG . Korea's Thyroid-Cancer ‘Epidemic’ — Screening and Overdiagnosis. N Engl J Med 2014;371:1765–1767.
Mehrzad R, Nishino M, Connolly J et al. The relationship between the follicular variant of papillary thyroid cancer and follicular adenomas. Surgery 2016;159:1396–1406.
Albores-Saavedra J, Henson DE, Glazer E et al. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype—papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol 2007;18:1–7.
Jung CK, Little MP, Lubin JH et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab 2014;99:E276–E285.
Nikiforov YE, Seethala RR, Tallini G et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol 2016;2:1023–1029.
Liu Z, Zhou G, Nakamura M et al. Encapsulated follicular thyroid tumor with equivocal nuclear changes, so-called well-differentiated tumor of uncertain malignant potential: a morphological, immunohistochemical, and molecular appraisal. Cancer Sci 2011;102:288–294.
Wallander M, Layfield LJ, Jarboe E et al. Follicular variant of papillary carcinoma: reproducibility of histologic diagnosis and utility of HBME-1 immunohistochemistry and BRAF mutational analysis as diagnostic adjuncts. Appl Immunohistochem Mol Morphol 2010;18:231–235.
Elsheikh TM, Asa SL, Chan JK et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol 2008;130:736–744.
Rosai J . The encapsulated follicular variant of papillary thyroid carcinoma: back to the drawing board. Endocr Pathol 2010;21:7–11.
Ganly I, Wang L, Tuttle RM et al. Invasion rather than nuclear features correlates with outcome in encapsulated follicular tumors: further evidence for the reclassification of the encapsulated papillary thyroid carcinoma follicular variant. Hum Pathol 2015;46:657–664.
Vivero M, Kraft S, Barletta JA . Risk stratification of follicular variant of papillary thyroid carcinoma. Thyroid 2013;23:273–279.
Thompson LD . Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: A name change to Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features would help prevent overtreatment. Mod Pathol 2016;29:698–707.
Lee KD, Lee HS . Central neck dissection for papillary thyroid carcinoma. J Korean Thyroid Assoc 2014;7:140.
Di Cristofaro J, Marcy M, Vasko V et al. Molecular genetic study comparing follicular variant versus classic papillary thyroid carcinomas: association of N-ras mutation in codon 61 with follicular variant. Hum Pathol 2006;37:824–830.
Lee SR, Jung CK, Kim TE et al. Molecular genotyping of follicular variant of papillary thyroid carcinoma correlates with diagnostic category of fine-needle aspiration cytology: values of RAS mutation testing. Thyroid 2013;23:1416–1422.
Arora N, Scognamiglio T, Lubitz CC et al. Identification of borderline thyroid tumors by gene expression array analysis. Cancer 2009;115:5421–5431.
Hwang TS, Kim WY, Preoperative RAS . mutational analysis is of great value in predicting follicular variant of papillary thyroid carcinoma. Biomed Res Int 2015;2015:697068.
Rivera M, Ricarte-Filho J, Knauf J et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol 2010;23:1191–1200.
Howitt BE, Jia Y, Sholl LM et al. Molecular alterations in partially-encapsulated or well-circumscribed follicular variant of papillary thyroid carcinoma. Thyroid 2013;23:1256–1262.
Lupi C, Giannini R, Ugolini C et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab 2007;92:4085–4090.
McFadden DG, Dias-Santagata D, Sadow PM et al. Identification of oncogenic mutations and gene fusions in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab 2014;99:E2457–E2462.
Rossi ED, Martini M, Bizzarro T et al. Uncommon BRAF mutations in the follicular variant of thyroid papillary carcinoma: New insights. Cancer Cytopathol 2015;123:593–602.
Howitt BE, Paulson VA, Barletta JA . Absence of BRAF V600E in non-infiltrative, non-invasive follicular variant of papillary thyroid carcinoma. Histopathology 2015;67:579–582.
Asa SL, de Jesus AC, Kerr D et al. Thyroid. Chapter 13 In: Mete O, Asa SL (eds). Endocrine Pathology. Cambridge University Press: Cambridge, UK, 2016, pp 398–572.
Jung CK, Im SY, Kang YJ et al. Mutational patterns and novel mutations of the BRAF gene in a large cohort of Korean patients with papillary thyroid carcinoma. Thyroid 2012;22:791–797.
Oh WJ, Lee YS, Cho U et al. Classic papillary thyroid carcinoma with tall cell features and tall cell variant have similar clinicopathologic features. Korean J Pathol 2014;48:201–208.
Cho U, Oh WJ . Clinicopathological features of rare BRAF mutations in Korean thyroid cancer patients. J Korean Med Sci 2014;29:1054–1060.
den Dunnen JT, Antonarakis SE . Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 2000;15:7–12.
Yeo MK, Bae JS, Oh WJ et al. Macrofollicular variant of papillary thyroid carcinoma with extensive lymph node metastases. Endocr Pathol 2014;25:265–272.
Haugen BR, Alexander EK, Bible KC et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2015;26:1–133.
Min HS, Lee C, Jung KC . Correlation of immunohistochemical markers and BRAF mutation status with histological variants of papillary thyroid carcinoma in the Korean population. J Korean Med Sci 2013;28:534–541.
Chen H, Izevbaye I, Chen F et al. Recent advances in follicular variant of papillary thyroid carcinoma. North Am J Med Sci 2012;5:212–216.
Fagin JA, Wells SA Jr . Biologic and Clinical Perspectives on Thyroid Cancer. N Engl J Med 2016;375:1054–1067.
Acknowledgements
This research was supported by a grant (2013R1A2A2A01068570) of Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future planning, and a grant (HI16C2013) of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cho, U., Mete, O., Kim, MH. et al. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol 30, 810–825 (2017). https://doi.org/10.1038/modpathol.2017.9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2017.9
This article is cited by
-
Differentiating BRAF V600E- and RAS-like alterations in encapsulated follicular patterned tumors through histologic features: a validation study
Virchows Archiv (2024)
-
Nodal metastasis in noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)
Endocrine (2024)
-
Is it Possible to Diagnose “Non-Invasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features” Preoperatively?
Indian Journal of Surgical Oncology (2023)
-
The Presence of Typical “BRAFV600E-Like” Atypia in Papillary Thyroid Carcinoma is Highly Specific for the Presence of the BRAFV600E Mutation
Endocrine Pathology (2023)
-
Hemithyreoidektomie oder totale Thyreoidektomie beim papillären Niedrigrisikokarzinom der Schilddrüse?
Die Chirurgie (2023)