Abstract
Thrombospondin type I domain-containing 7A (THSD7A) is a known antigenic target of autoantibodies leading to primary membranous glomerulopathy and was reported to account for ~10% of phospholipase A2 receptor (PLA2R)-negative membranous glomerulopathy. It has been proposed that PLA2R and THSD7A autoantibodies are mutually exclusive in membranous glomerulopathy. We validated an immunohistochemical assay to investigate for THSD7A-associated membranous glomerulopathy and utilized it in 258 consecutive native kidney biopsies, which showed membranous glomerulopathy in our laboratory, with the exception of membranous lupus nephritis. Membranous glomerulopathy stained positive for THSD7A-only in 7 (3%) cases, PLA2R-only in 141 (55%) cases, and showed dual positivity for THSD7A and PLA2R in 2 (1%) cases. Serologic testing for antibodies to PLA2R and THSD7A was performed in a subset of these patients. There was 100% correlation between positive THSD7A and/or PLA2R tissue staining and the presence of the corresponding autoantibodies in the serum including the two cases with dual positive THSD7A and PLA2R antibodies. We describe and provide a protocol for detection of THSD7A-associated membranous glomerulopathy in clinical practice. The cases with dual THSD7A and PLA2R positivity show that these autoantibodies are not mutually exclusive. They also emphasize the importance of using a panel-based approach when subtyping membranous glomerulopathy as a patient could conceptually be identified and treated based on anti-PLA2R titers, but still have anti-THSD7A antibodies driving persistent disease.
Similar content being viewed by others
Main
Primary membranous glomerulopathy is an autoimmune disease in which autoantibodies are directed against podocyte antigens resulting in subepithelial immune deposits and nephrotic syndrome. The antigenic target of these autoantibodies was unknown until 2009 when phospholipase A2 receptor (PLA2R) was described as the antigenic target in ~70% of cases with primary membranous glomerulopathy.1 This discovery was quickly translated to the clinic and, today, renal biopsy staining for PLA2R allows for a specific diagnostic classification of the type of membranous glomerulopathy present.2, 3 In addition, serum autoantibodies to PLA2R have been shown to be a useful biomarker for monitoring disease activity.4, 5
Thrombospondin type-1 domain-containing 7A (THSD7A) was recently described as a second antigenic target in primary membranous glomerulopathy.6 In that initial case series, ~10% of membranous glomerulopathy patients negative for PLA2R autoantibodies instead had circulating THSD7A autoantibodies. It has been proposed that PLA2R and THSD7A are mutually exclusive.6, 7 We validated an assay for the detection of THSD7A-associated membranous glomerulopathy and compared pathologic findings in this variant to those of PLA2R-associated membranous glomerulopathy. In the process, we identified two cases with dual PLA2R and THSD7A autoantibodies.
Materials and methods
Patient Selection
Immunohistochemical staining for THSD7A was performed on all kidney biopsies, in which PLA2R was ordered in routine clinical care between December 2014 and April 2015. This included all native kidney biopsies that showed membranous glomerulopathy seen in our practice during this time period with the exception of membranous lupus nephritis (ISN/RPS class V), a total of 258 cases. This study was approved by Schulman Associates institutional review board.
Standard Renal Biopsy Processing Techniques
Standard renal biopsy processing techniques were used including light, immunofluorescence, and electron microscopy.8, 9 All light microscopy samples were stained with hematoxylin and eosin, Jones methenamine silver, Masson trichrome, and periodic acid-Schiff reagent. All direct immunofluorescence sections were cut at 5 μm and reacted with fluorescein-tagged polyclonal rabbit anti-human antibodies to IgG, IgA, IgM, C3, C1q, fibrinogen, and κ- and λ-light chains (Dako, Carpenteria, CA, USA) for 1 h, rinsed, and a coverslip applied using the aqueous mounting media. For electron microscopy, thin sections were examined in a Jeol JEM-1011 electron microscope (Jeol, Tokyo, Japan). Photomicrographs were routinely taken at × 5000, × 12 000, and × 20 000 magnifications.
PLA2R1 Immunofluorescence
The PLA2R1 immunofluorescence staining procedure was performed as previously described.2 Briefly, PLA2R1 was detected in paraffin embedded sections using rabbit polyclonal anti-PLA2R1 antibodies (Sigma–Aldrich) at a dilution of 1:50 followed by highly cross-adsorbed Alexa Fluor 488 goat anti-rabbit IgG (Life Technologies, Carlsbad, CA, USA) at a dilution of 1:100. Each case was run with a positive and negative (secondary antibody only) control. The stain was evaluated by standard immunofluorescence microscopy using a Leica L5 filtercube. It was judged to be positive if there was positive granular capillary loop staining in the glomeruli and negative if there was no staining in glomeruli.
THSD7A Immunohistochemistry
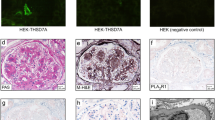
The THSD7A immunohistochemical stain was performed on a Dako Autostainer Link 48 as detailed in Table 3. Since THSD7A is normally present in podocytes, the aim was to optimize the method, such that we had minimal THSD7A staining in normal glomeruli and non-THSD7A membranous glomerulopathy while maintaining strong positive staining in THSD7A-associated membranous glomerulopathy. The staining pattern of THSD7A was scored in all membranous glomerulopathy cases. Glomerular staining along the glomerular basement membranes was commonly present. However, strong diffuse global granular staining of THSD7A along the capillary loops was only present in the THSD7A-associated membranous glomerulopathy cases (Figure 1).
Immunoperoxidase staining for THSD7A in membranous glomerulopathy. (a) THSD7A staining in a normal glomerulus shows weak segmental staining of the glomerular basement membranes. Original magnification × 400. (b and c): Glomeruli from two different cases of PLA2R-associated membranous glomerulopathy demonstrate the range of normal THSD7A staining that can be seen in cases of membranous glomerulopathy from (b) weak segmental staining along the glomerular basement membranes to (c) a global moderate pseudo-linear pattern. Original magnification × 400. (d) Strong global granular capillary loop staining is evident in this case of THASD7A-associated membranous glomerulopathy. Original magnification × 400.
Western Blot Analysis
Human glomerular extract and recombinant PLA2R1 were prepared as previously described.1 Full-length human THSD7A, and constructs encoding the cysteine-rich region (CysR; amino acids 26–170) and the first C-type lectin-like domain (CTLD1; amino acids 221–356) of human PLA2R1 were expressed in HEK293 cells and the cell extract (THSD7A) or the conditioned medium (PLA2R CysR and CTLD1) used for Western blotting. Gel electrophoresis and protein transfer to nitrocellulose membranes were performed according to standard protocols, and blots were blocked with 10% milk in Tris-buffered normal saline-Tween 20 (TBS-T). For use as the primary antibody, patient serum was diluted at a 1:100 ratio and incubated overnight at 4 °C. IgG4 specific autoantibodies were detected with sheep anti-human IgG4 (1:3000; The Binding Site), followed by peroxidase-conjugated donkey anti-sheep IgG (1:10 000; Jackson ImmunoResearch). The blots were incubated in chemiluminescent substrate, then exposed to photographic film for 5 s to 4 min and developed in a Kodak X-Omat developer.
Results
We sought to validate a staining technique for the detection of THSD7A-associated membranous glomerulopathy on kidney biopsies. An immunoperoxidase staining method was utilized as our initial attempt using an indirect immunofluorescence technique, similar to that described for PLA2R,10 showed false negative staining. Since THSD7A is normally present in podocytes, the aim was to optimize the method such that we had minimal THSD7A staining in normal glomeruli and non-THSD7A membranous glomerulopathy while maintaining strong positive staining in THSD7A-associated membranous glomerulopathy. Weak pseudo-linear staining along the glomerular basement membranes was commonly present under normal conditions and in non-THSD7A-associated membranous glomerulopathy. However, strong diffuse global granular staining of THSD7A along the capillary loops was only present in the THSD7A-associated membranous glomerulopathy cases (Figure 1).
Membranous glomerulopathy stained positive for THSD7A-only in 7 (3%) cases, PLA2R-only in 141 (55%) cases, and showed dual positivity for THSD7A and PLA2R in 2 (1%) cases (Figure 2). Staining was negative for both in 108 (42%) cases. A summary of the clinical findings for the nine patients with THSD7A-positive membranous glomerulopathy is available in Table 1. They had a mean age of 62 years and a mean of 8.2 g of proteinuria per day. Serologic tests for ANA, ANCA, HBV, HCV, and HIV as well as complement levels were normal in all of these patients. One of the patients had a remote history of membranous glomerulopathy that was in clinical remission for 7 years prior to the current biopsy. Tissue from the previous biopsy was not available for examination.
Membranous glomerulopathy with dual positive staining. (a and b) This case of membranous glomerulopathy showed strong global granular capillary wall staining for both (a) PLA2R (indirect immunofluorescence; original magnification × 400) and (b) THSD7A (immunoperoxidase; original magnification × 400).
Serologic testing for antibodies to PLA2R and THSD7A was performed in four of the THSD7A-positive patients who had serum available from the time of biopsy. We also tested serum from five membranous glomerulopathy cases with PLA2R-only staining, one membranous glomerulopathy case negative for both PLA2R and THSD7A, and one case without membranous glomerulopathy on biopsy for comparison. There was 100% correlation between positive THSD7A and/or PLA2R tissue staining and the presence of the corresponding autoantibodies in the serum including the two cases with dual positive THSD7A and PLA2R antibodies (Table 2 and Figure 3).
Western blotting with patient serum to detect reactivity against PLA2R and THSD7A. (a) Sera were initially screened by Western blotting against human glomerular extract (HGE) and cell-expressed recombinant PLA2R. Several sera (1 and 3) react with a band in HGE that did not appear to be PLA2R. (b) Representative sera are blotted against both recombinant PLA2R and THSD7A. For case 1 (and case 3, not shown) the reactivity corresponding to the band seen in HGE in panel A is confirmed to be against THSD7A. Immunoblotting against both antigens shows that case 4 has reactivity to both PLA2R and THSD7A. Repeated use of a very limited amount of the initial serum sample explains the weak PLA2R signal for case 4. A follow-up (4f/u) serum was obtained that clearly shows reactivity with both antigens. (c) To confirm reactivity with PLA2R, sera were additionally tested by Western blot against the immunodominant epitope, which resides in CysR,14 as well as a minor epitope in CTLD1. Although none of the cases had reactivity to CTLD1, the anti-PLA2R only cases (2 and 5) and the dual anti-PLA2R and anti-THSD7A case (4) exhibited reactivity with the CysR domain of PLA2R. P-T+, anti-PLA2R negative and anti-THSD7A positive; P+T-, anti-PLA2R positive and anti-THSD7A negative; P+T+ dual positive for anti-PLA2R and anti-THSD7A.
Renal biopsy findings in cases of THSD7A-associated membranous glomerulopathy were typical of that which is present in primary membranous glomerulopathy. Immunofluorescence was positive for IgG, C3, κ, and λ in all cases without IgA, IgM, or C1q staining. Electron microscopy in all nine cases showed evidence of subepithelial deposits without subendothelial or mesangial deposits. There was no evidence of IgG staining outside of the glomeruli such as the tubular basement membranes. IgG subtype analysis was performed in six of the nine THSD7A positive cases. There were three cases co-dominant for IgG1 and IgG4, two cases were IgG1 dominant, and one case was IgG4 dominant.
Discussion
The diagnosis of membranous glomerulopathy is made when the renal biopsy shows a combination of granular deposits of IgG along the glomerular basement membranes on immunofluorescence in combination with subepithelial deposits by electron microscopy. Traditionally, this disease has been divided into primary, in which the etiology is unknown, and secondary when one of numerous associated conditions is identified. Identification of PLA2R as the antigenic target in ~70% of primary membranous glomerulopathy has made it possible to diagnose a specific subtype of membranous glomerulopathy based on the underlying pathogenic mechanism driving the disease and has enabled non-invasive monitoring utilizing serologic testing for PLA2R antibodies as biomarkers of activity.4, 5 The recent identification of THSD7A as a second antigenic target of antibodies leading to primary membranous glomerulopathy provides the ability to classify more of these cases. We provide here our experience with detecting THSD7A-associated membranous glomerulopathy on renal biopsy using a specific immunohistochemical stain in a large biopsy cohort.
Subclassifying membranous glomerulopathy based on the autoantibody driving the disease can be performed through serologic testing for autoantibodies or staining the renal biopsy. In the setting of PLA2R, tissue staining has been shown to be more sensitive for the detection of the type of membranous glomerulopathy present than serum testing.11 Care must be taken when utilizing an immunoperoxidase technique for membranous subtyping to avoid mistaking normal podocyte staining for evidence of disease-associated positivity in glomeruli (Figure 1). We have found that for THSD7A, a diffuse global capillary wall staining pattern for THSD7A is specific for identifying cases in which the patients have autoantibodies directed against this protein. The protocol utilized in our laboratory is provided in Table 3. However, careful validation must be performed in each laboratory as variability in local tissue processing protocols (eg, fixation type and time) might affect these results and alterations in the protocol will likely be necessary to achieve acceptable results.
We present here the first report of cases of membranous glomerulopathy with dual positive antibodies against THSD7A and PLA2R. These rare cases with dual THSD7A and PLA2R positivity show that these autoantibodies are not mutually exclusive and reveal the need for further study to determine the underlying etiology when more than one antibody is present. We do not know at this time if both autoantibodies are pathogenic in driving disease. It is possible that one of the autoantibodies represents ‘opportunistic’ antibody formation after podocyte antigens are exposed in disease. Alternatively, as genetic association studies have shown that the genetic susceptibility to idiopathic membranous glomerulopathy involves HLA DQA1,12, 13 it is possible that certain individual’s immune systems are genetically primed to produce autoantibodies to podocyte antigens and both are pathogenic.
The diagnosis and treatment of membranous glomerulopathy is in the midst of transformation as details concerning the underlying pathogenic drivers of disease are uncovered. We present here our experience with THSD7A staining of renal biopsies in clinical practice. Further work is needed to determine whether or not serologic studies for THSD7A are useful for noninvasive monitoring of disease activity as they are for PLA2R. Unlike previous reports, we show that THSD7A and PLA2R autoantibodies do rarely occur together in cases of membranous glomerulopathy. These cases emphasize the importance of using a panel-based approach when subtyping membranous glomerulopathy as a patient could conceptually be identified and treated based on anti-PLA2R titers, but still have anti-THSD7A antibodies driving persistent disease.
References
Beck LH, Bonegio RG, Lambeau G et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009;361:11–21.
Larsen CP, Messias NC, Silva FG et al. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor (PLA2R1) staining in renal biopsies. Mod Pathol 2013;26:709–715.
Hoxha E, Harendza S, Zahner G et al. An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 2011;26:2526–2532.
Ruggenenti P, Debiec H, Ruggiero B et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 2015;26:2545–2458.
Hoxha E, Thiele I, Zahner G et al. Phospholipase A2 receptor autoantibiodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 2014;25:1357–1366.
Tomas NM, Beck LHJ, Meyer-Schwesinger C et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014;371:2277–2287.
Iwakura T, Ohashi N, Kato A et al. Prevalence of enhanced granular expression of thrombospondin type-1 domain-containing 7A in the glomeruli of Japanese patients with idiopathic membranous nephropathy. PLoS One 2015;10:e0138841.
Walker PD, Cavallo T, Bonsib SM . Practice guidelines for the renal biopsy. Mod Pathol 2004;17:1555–1563.
Walker PD . The renal biopsy. Arch Pathol Lab Med 2009;133:181–188.
Larsen CP, Beggs ML, Walker PD et al. Histopathologic effect of APOL1 risk alleles in PLA2R-associated membranous glomerulopathy. Am J Kidney Dis 2014;64:156–163.
Debiec H, Ronco P . PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 2011;364:689–690.
Stanescu HC, Arcos-Burgos M, Medlar A et al. Risk HLA-DQA1 and PLA2R1 alleles in idiopathic membranous nephropathy. N Engl J Med 2011;364:616–626.
Saeed M, Beggs ML, Walker PD et al. PLA2R-associated membranous glomerulopathy is modulated by common variants in PLA2R1 and HLA-DQA1 genes. Genes Immun 2014;15:556–561.
Fresquet M, Jowitt TA, Gummadova J et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol 2015;26:302–313.
Acknowledgements
LHB is supported by NIH/NIDDK R01 DK097053.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CPL and LNC declare no competing interests. LHB is a co-inventor on the US patent ‘Diagnostics for Membranous Nephropathy’.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Larsen, C., Cossey, L. & Beck, L. THSD7A staining of membranous glomerulopathy in clinical practice reveals cases with dual autoantibody positivity. Mod Pathol 29, 421–426 (2016). https://doi.org/10.1038/modpathol.2016.32
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.32