Abstract
T1 colorectal cancer can be mimicked by pseudo-invasion in pedunculated polyps. British guidelines are currently one of the few which recommend diagnostic confirmation of T1 colorectal cancer by a second pathologist. The aim of this study was to provide insights into the accuracy of histological diagnosis of pedunculated T1 colorectal cancer in daily clinical practice. A sample of 128 cases diagnosed as pedunculated T1 colorectal cancer between 2000 and 2014 from 10 Dutch hospitals was selected for histological review. Firstly, two Dutch expert gastrointestinal pathologists reviewed all hematoxylin-eosin stained slides. In 20 cases the diagnosis T1 colorectal cancer was not confirmed (20/128; 16%). The discordant cases were subsequently discussed with a third Dutch gastrointestinal pathologist and a consensus diagnosis was agreed. The revised diagnoses were pseudo-invasion in 10 cases (10/128; 8%), high-grade dysplasia in 4 cases (4/128; 3%), and equivocal in 6 cases (6/128; 5%). To further validate the consensus diagnosis, the discordant cases were reviewed by an independent expert pathologist from the United Kingdom. A total of 39 cases were reviewed blindly including the 20 cases with a revised diagnosis and 19 control cases where the Dutch expert panel agreed with the original reporting pathologists diagnosis. In 19 of the 20 cases with a revised diagnosis the British pathologist agreed that T1 colorectal cancer could not be confirmed. Additionally, amongst the 19 control cases the British pathologist was unable to confirm T1 colorectal cancer in a further 4 cases and was equivocal in 3 cases. In conclusion, both generalist and expert pathologists experience diagnostic difficulty distinguishing pseudo-invasion and high-grade dysplasia from T1 colorectal cancer. In order to prevent overtreatment, review of the histology of pedunculated T1 colorectal cancers by a second pathologist should be considered with discussion of these cases at a multidisciplinary meeting.
Similar content being viewed by others
Main
Population-based colorectal cancer screening programs have been widely introduced in Western countries.1, 2 The four main difficulties in the pathology of the screening program have been reported to be the recognition of serrated lesions, a biopsy diagnosis of malignancy, determining risk features in T1 colorectal cancer, and the recognition of pseudo-invasion.3, 4 The latter, also called epithelial misplacement or pseudo-carcinomatous invasion, has been advocated to be the pathologic diagnostic conundrum of the screening program with important consequences.4 In contrast to T1 colorectal cancer, pseudo-invasion is a benign condition that lacks metastatic potential. Therefore, inadequate recognition can lead to over diagnosis and the subsequent risk of surgical overtreatment of benign lesions.4
Pseudo-invasion was first described in 1973 by Muto et al.5 It is a phenomenon in which cancer is mimicked in a benign lesion by misplacement of epithelium from the mucosa to the submucosa, and rarely even in the muscularis propria.6, 7 As it is mostly seen in pedunculated polyps located in the sigmoid colon, it is hypothesized that it is caused by tension and shear forces leading to mucosal prolapse, microtrauma, and subsequent breaks in the muscularis mucosae.8, 9, 10 Pedunculated sigmoidal polyps are exposed to enhanced trauma due to the narrow lumen in which solid feces passes, the curves the sigmoid colon makes and the relatively loosely attached mucosa. These forces can result in epithelial necrosis and subsequent hemorrhage, which can be identified by hemosiderin deposition.
The United Kingdom guideline on colorectal cancer screening is currently one of the few that recommends diagnostic confirmation of T1 colorectal cancer by a second pathologist.3, 11 This recommendation is not yet widely followed by other guidelines, as it is currently unknown on what scale misclassification takes place in daily clinical practice. The aim of this study was to provide insights in the diagnostic accuracy and reproducibility of the histological diagnosis of pedunculated T1 colorectal cancers in daily clinical practice, by review of the original histological diagnosis by pathologists with special expertize in gastrointestinal pathology.
Materials and methods
Study Population and Sample Collection
This study was conducted in a sub-cohort of the T1 colorectal cancer registration cohort, initiated by the Dutch T1 Colorectal Cancer Working Group. This collaboration initiative consists of 10 hospitals, including 9 non-academic and 1 academic hospital. All patients with T1 colorectal cancer diagnosed between 2000 and 2014 in these hospitals were identified using the Netherlands Cancer Registry. The electronic medical records of all patients were reviewed. Only cases in which the local pathologist clearly confirmed the diagnosis pT1 colorectal cancer in the pathology report were included, which was defined as tumors with invasion through the muscularis mucosa and into, but not beyond, the submucosa. Patients with hereditary predisposition for colorectal cancer, inflammatory bowel disease, synchronous advanced colorectal cancer, non-cancer related death within one year after treatment, and non-pedunculated or unknown morphology were excluded. Patient and polyp characteristics were collected. In addition, follow-up data were collected in order to correlate the diagnosis after review to the outcome during follow-up.
A sample of cases was selected for histological review. Selection was based on inclusion in another study (not published yet). For that study, the hematoxylin-eosin stained slides of all pedunculated T1 colorectal cancer cases with either lymph node metastasis or recurrent cancer were collected from the participating hospitals. In addition, hematoxylin-eosin stained slides of matched pedunculated T1 colorectal cancer cases without lymph node metastasis or recurrent cancer were collected from the same hospitals. Matching was performed based on type of treatment and polyp location using the method of incidence density sampling. Collection of the slides and matching was performed by two independent researchers who were not involved in the review process (YB and LM). Slides were scrambled by the same two researchers before pathology review, so that the participating pathologists remained fully blinded.
This study was approved by the Medical Ethics Review Committee of the University Medical Center Utrecht (approval for data-collection, reference number: 15–487; approval for histological review, reference number 15–716). The study was performed in accordance with the Helsinki Declaration. All patients’ data were coded and anonymity of patients was guaranteed.
Pathology Review
Three pathologists from the Netherlands (ML, AM, and JO) and a pathologist from the United Kingdom (MN) participated in this study, all with special expertize in gastrointestinal pathology. All pathologists were blinded to the clinical characteristics (ie, patient and polyp characteristics) and clinical outcome (ie, lymph node metastasis or recurrent cancer during follow-up). In order to guarantee the same setting for the original pathologists and the pathologists that performed the review, all available hematoxylin-eosin stained slides were provided for reading; no additional deeper sections were performed.
A flowchart of the study design is presented in Figure 1. First, all slides on which the local pathologist diagnosed T1 colorectal cancer were mutually reviewed by two pathologists from the Netherlands (JO and ML). In this review, the pathologists decided together whether they could confirm the original diagnosis of T1 colorectal cancer. Then, a consensus meeting was held comprising a panel of three pathologists (JO, ML, and AM). In this consensus meeting, all cases in which the diagnosis of T1 colorectal cancer was not confirmed following the review process were discussed by the three pathologists together. Equivocal was scored when T1 colorectal cancer could not be excluded but could not be confirmed either. When the initial diagnosis of T1 colorectal cancer was not confirmed, the expert panel specified whether the revised diagnosis was low-grade dysplasia, high-grade dysplasia or pseudo-invasion. This led to a majority consensus histological diagnosis applying a two out of three agreement method.
In order to validate the consensus diagnoses, all cases in which the diagnosis of T1 colorectal cancer was not confirmed at the consensus meeting were independently reviewed by a fourth pathologist from the United Kingdom with extensive experience of difficult bowel cancer screening program polyp histology (MN). In order to ensure cases were reported blindly an additional 19 cases, where the consensus diagnosis agreed with the original diagnosis, were mixed with the cases with revised diagnoses.
Histological Definitions
The term dysplasia was used to indicate the presence of intra-epithelial neoplastic proliferation, graded according to the Vienna classification of gastrointestinal epithelial neoplasia.12 Pseudo-invasion was defined as misplacement of epithelium from the mucosa to the submucosa or deeper.6, 7 In all cases with a revised diagnosis of pseudo-invasion, characteristics supporting the diagnosis of pseudo-invasion as described and defined by Loughrey et al were scored by two pathologists (ML and JO).3 T1 colorectal cancer was defined as polyps with invasion through the muscularis mucosa and into, but not beyond, the submucosa.13 Characteristics supporting the diagnosis T1 colorectal cancer (ie, lymphovascular invasion and tumor budding) were scored by the same two pathologists (ML and JO). Lymphovascular invasion was defined as the presence of cancer cells within endothelial-lined channels.14 Tumor budding was defined, measured and scored according to the method proposed by Ueno et al.15
Statistical Analysis
Categorical data were expressed as frequencies and percentages, continuous variables as medians with their interquartile ranges. Primary endpoint of the study was the agreement of pathological diagnosis of pedunculated T1 colorectal cancer of the original pathologist with the second review. Agreement concerning the diagnosis of T1 colorectal cancer was expressed in percentages. Bootstrapping was performed to calculate 95% confidence intervals. A sub-analysis was performed in order to explore whether the percentage of discordant diagnoses had improved in the latest years. The percentage of cases in which the original diagnosis of T1 colorectal cancer was not confirmed was compared between patients diagnosed before 2011 and patients diagnosed from 2011 onwards using the Pearson's X2-test. In addition, clinicopathological characteristics of discordant cases and agreement cases were compared using the Pearson's X2-test or Mann–Whitney U-test as appropriate. A two-sided P-value ≤0.05 was considered statistically significant. SPSS Statistics version 21 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Study Population
In total, 457 cases of pedunculated T1 colorectal cancer were identified in the participating hospitals (Figure 2). Hematoxylin-eosin stained slides of a sample of 128 cases were collected for histological review. Median age of the revised cohort was 67 years (61–74) with 53% male (Table 1). Median polyp size was 20 millimeter (15–30), with the majority of polyps located in the sigmoid colon (72%). Median follow-up time of the patients was 47 months (27–79).
Agreement of Review with Original Diagnosis
During review, the original diagnosis of T1 colorectal cancer was not confirmed in 20 cases (16%; 95% confidence interval 10–22%). In the consensus meeting, three-way agreement on the final diagnosis was reached in 95% (19/20) of cases. The revised diagnosis was pseudo-invasion in 10 cases (8%; 95% confidence interval 4–13%), high-grade dysplasia in 4 cases (3%; 95% confidence interval 1–6%) and equivocal in 6 cases (5%; 95% confidence interval 2–9%). In a sub-analysis of cases diagnosed before and after 2011, the original diagnosis of T1 colorectal cancer was not confirmed in 15% (13/88) of the cases diagnosed between 2000 and 2010 vs 18% (7/40) of the cases diagnosed from 2011 onwards, P=0.69.
Clinicopathological Characteristics of Discordant Cases
Cases with a revised diagnosis were compared with the agreement cases with regard to clinicopathological characteristics (Table 2). Lymph node metastasis or recurrent cancer during follow-up were not observed in any of the patients where the consensus panel downgraded the diagnosis of T1 colorectal cancer. The reviewed diagnosis was significantly more often in disagreement with the original diagnosis in polyps located in the sigmoid colon vs other locations (20 vs 6%, P=0.05). No difference with regard to polyp size was observed between the groups. In none of the discordant cases tumor budding or lymphovascular invasion was observed.
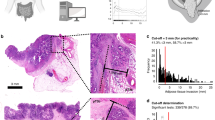
In the 10 cases with a revised diagnosis of pseudo-invasion, characteristics supporting the diagnosis of pseudo-invasion were scored (Table 3). Features present in all 10 cases included epithelial differentiation similar to that of the surface epithelium, fibromuscular stromal proliferation and mucosal prolapse changes. Accompaniment by non-adenomatous epithelium was least often seen, only in 1 case. Examples of the features are presented in Figure 3.
Examples of pseudo-invasion cases. (a, b) Overview and detail. Mucosal prolapse changes, haemosiderin deposition and dilated cystic glands. (c, d) Overview and detail. Haemosiderin deposition, mucus cysts and fibromuscular stromal proliferation. (e, f) Overview and detail. Dilated cystic glands and fibromuscular stromal proliferation.
Validation of Consensus Diagnosis by Independent Expert Pathologist
The hematoxylin-eosin stained slides of the cases in which review did not confirm the original diagnosis of T1 colorectal cancer were reviewed by a fourth expert gastrointestinal pathologist from the United Kingdom mixed with a random sample of 19 confirmed T1 colorectal cancer cases (Figure 1). In 19 of the 20 cases in which T1 colorectal cancer was not confirmed in the consensus meeting the fourth pathologist independently agreed (Table 4). In nine cases the mutual revised diagnosis was pseudo-invasion with high-grade dysplasia in a further three cases.
In 12 of the 19 other cases of this set in which the Dutch pathologists had agreed with the original diagnosis of T1 colorectal cancer, the fourth pathologist independently agreed. However, in the remaining 7 cases the fourth pathologist revised the diagnosis as either pseudo-invasion (N=3), high-grade dysplasia (N=1), or equivocal (N=3).
Discussion
In this large multicenter cohort, we observed that in 16% of patients initially diagnosed with pedunculated T1 colorectal cancer the diagnosis was not confirmed by a Dutch expert gastrointestinal pathology team. Most often the revised diagnosis was pseudo-invasion (8%). We validated the consensus diagnosis by a fourth independent expert gastrointestinal pathologist and noted that in 95% (19/20) of the cases all four pathologists agreed that the diagnosis T1 colorectal cancer could not be confirmed. Nevertheless, even among experienced gastrointestinal pathologists no universal agreement was observed, underlining the diagnostic difficulty of these cases. Based on these results, a second histological opinion seems advisable for cases of pedunculated T1 colorectal cancer, especially when surgery is considered.
Over diagnosis of T1 colorectal cancer has several important consequences. First, it may have clinical consequences. In our study, 55% of the patients in which the Dutch expert panel did not confirm the diagnosis had undergone secondary surgical resection (11/20, data not shown). Importantly, oncological colorectal surgery carries significant risks, with mortality percentages increasing from 4% in the elderly up to 10% in the oldest age groups.16, 17, 18 Also, frequency of endoscopic follow-up of non-invasive polyps differs from colorectal cancer and thus adequate recognition further eliminates overuse of valuable endoscopic resources. Second, over diagnosis pollutes cancer registries with false incidence rates and affects the prognosis data of early colorectal cancers. As can be seen in Figure 2, 40% of our cohort consists of polyps with a pedunculated morphology. Given the high percentage of non-confirmed T1 colorectal cancers observed in this study the incidence of ‘true’ pedunculated T1 colorectal cancers is almost certainly lower, and raises the possibility that the prognosis of ‘true’ pedunculated T1 colorectal cancers is probably worse than currently registered in the Netherlands Cancer Registry. Finally, over diagnosis may also have ethical consequences given that patients are erroneously burdened with a diagnosis of cancer.
The diagnostic difficulty of differentiating pseudo-invasion from true invasion might cause that disagreement will remain even among experts and a golden-standard diagnosis will not always be reached. In our cohort, 5% of cases resulted in an equivocal diagnosis after review by the Dutch consensus panel. Moreover, disagreement between the Dutch consensus panel and the British expert pathologist on the diagnosis of T1 colorectal cancer was observed. Discrepant diagnoses among experts have also been reported by the Bowel Cancer Screening Program Expert Board from the United Kingdom comprising three experienced pathologists.4 Of all referrals containing all kind of polyps, 3% was judged too ambiguous to arrive at a final consensus diagnosis. In our opinion, it is therefore of utmost importance that these difficult cases are discussed in multidisciplinary meetings. In this way, other factors such as polyp location, patient age, comorbidity and general condition might aid in weighing the decision whether or not to proceed to surgery.
Histological features could potentially assist pathologists in distinguishing pseudo-invasion from true invasion. The characteristics as scored in Table 3 point toward pseudo-invasion; however, none of them is pathognomonic.3, 4, 5 The presence of lymphovascular invasion confirms malignancy, and indeed was not observed in any of the downgraded cases. However, it must be emphasized that diagnosing lymphovascular invasion is prone to high interobserver variation too.19, 20, 21, 22 We observed that epithelial differentiation similar to that of the surface epithelium, fibromuscular stromal proliferation and mucosal prolapse changes were present in all cases with a revised diagnosis of pseudo-invasion. In the absence of lymphovascular invasion and in the presence of these three features pseudo-invasion should always be considered, keeping in mind that pseudo-invasion does not preclude true invasion elsewhere in the polyp.3
Several studies have assessed the value of immunohistochemical stains with collagen IV, E-cadherin, Matrix Metalloproteinase-1, p53, Ki67, urokinase Plasminogen Activating Receptor, α1 chain of type XI collagen, and stromelysin-3.9, 23, 24, 25, 26, 27 Thus far, none of them has proven to be a true reliable biomarker. The difficulty might be that in pseudo-invasive polyps the mucosal component can also show an enhanced immune-expression pattern, especially in polyps containing intramucosal carcinoma.9, 23
Although pseudo-invasion is a phenomenon most frequently described in pedunculated polyps, it has also been reported in sessile polyps after previous biopsy.6, 26, 28 Biopsy can cause disruption of the epithelium and herniation of glands and neoplastic cells into the submucosa. This was reported in one-third of the polyps previously biopsied, most often in sessile polyps of large size located in the proximal colon.26 Confinement of atypical epithelium to fibrin and granulation tissue may help to identify these polyps.26 Future studies are needed to shed light on the rate of misdiagnosis of T1 colorectal cancer in sessile polyps in order to explore whether a second opinion for this morphological type of T1 colorectal cancer is advisable as well.
The strengths of our study include the large number of pedunculated T1 colorectal cancer cases reviewed from multiple hospitals within the Netherlands. Clinical characteristics and follow-up characteristics were collected from all patients. Therefore, we were able to make an extra verification step by comparing the revised diagnosis with the presence or absence of lymph node metastasis and recurrent cancer during follow-up, and observed that in none of the downgraded cases lymph node metasisis or recurrent cancer was present. Participating pathologists from the Netherlands and the United Kingdom reviewed the samples blinded for the patient outcome and fully independent of each other. They have not worked together before or discussed this subject previously. Therefore, the diagnosis of the British pathologist can reliably be interpreted as a fully independent diagnosis.
Several limitations have to be acknowledged in this study. First, review of a sample of patients instead of the whole cohort could potentially have resulted in a biased incidence of discordant diagnoses given that due to the selection procedure a relatively high proportion of patients with an unfavorable outcome (lymph node metastasis or recurrent cancer) were reviewed. However, if bias had been introduced this would most likely have resulted in an underestimation of the incidence of misdiagnosed T1 colorectal cancers rather than an overestimation, given that patients with an unfavorable outcome (lymph node metastasis or recurrent cancer) have, by definition, a malignant diagnosis. Moreover, we verified whether the reviewed and non-reviewed cases had comparable baseline characteristics and observed no significant differences with regard to the clinical factors suggested to be associated with increased risk for pseudo-invasion in literature (ie, polyp size and location in sigmoid colon; Supplementary Material Table 1).4, 8, 9, 10 Thereby, the exact incidence of discordant diagnosis can probably never be estimated given that a golden-standard diagnosis does not exist. Secondly, patients in our study were diagnosed between 2000 and 2014. One might argue that the problem is of decreasing significance due to improved knowledge over time. However, we observed that even in the latest years (2011–2014), still 18% of diagnoses of T1 colorectal cancer were not confirmed. Moreover, the finding that to date even expert gastrointestinal pathologists did not agree on all cases, demonstrates that the diagnosis of pedunculated T1 colorectal cancer is a contemporary challenge.
In conclusion, the lack of agreement observed in this study on the diagnosis of T1 colorectal cancer in pedunculated polyps in a high percentage of cases underlines the difficulty of this diagnosis. Given that pathologists will encounter this problem with increasing frequency due to the introduction of colorectal cancer screening programs, it is of utmost importance that both gastroenterologists and pathologists are aware of this problem. Until a more objective marker is found, we strongly recommend the use of a majority consensus diagnosis in order to limit the risk of overtreatment of non-invasive adenomas, pollution of cancer registries and needless patient burden with the diagnosis cancer.
References
Altobelli E, Lattanzi A, Paduano R et al, Colorectal cancer prevention in Europe: burden of disease and status of screening programs. Prev Med 2014; 62: 132–141.
Singh R, Mangira D, Kawano H et al, Screening colonoscopy in Australia. Dig Endosc 2015; 27 (Suppl 1): 30–34.
Loughrey MB, Shepherd NA . The pathology of bowel cancer screening. Histopathology 2015; 66: 66–77.
Shepherd NA, Griggs RK . Bowel cancer screening-generated diagnostic conundrum of the century: pseudoinvasion in sigmoid colonic polyps. Mod Pathol 2015; 28 (Suppl 1): S88–S94.
Muto T, Bussey HJ, Morson BC . Pseudo-carcinomatous invasion in adenomatous polyps of the colon and rectum. J Clin Pathol 1973; 26: 25–31.
Magro G, Aprile G, Vallone G et al, Epithelial misplacement in the muscularis propria after biopsy of a colonic adenoma. Virchows Arch 2007; 450: 603–605.
Shepherd NA, Bussey HJ, Jass JR . Epithelial misplacement in Peutz-Jeghers polyps. A diagnostic pitfall. Am J Surg Pathol 1987; 11: 743–749.
Byun TJ, Han DS, Ahn SB et al, Pseudoinvasion in an adenomatous polyp of the colon mimicking invasive colon cancer. Gut Liver 2009; 3: 130–133.
Tanizawa T, Seki T, Nakano M et al, Pseudoinvasion of the colorectal polypoid tumors: serial section study of problematic cases. Pathol Int 2003; 53: 584–590.
Pascal RR, Hertzler G, Hunter S et al, Pseudoinvasion with high-grade dysplasia in a colonic adenoma. Distinction from adenocarcinoma. Am J Surg Pathol 1990; 14: 694–697.
Williams JG, Pullan RD, Hill J et al, Management of the malignant colorectal polyp: ACPGBI position statement. Colorectal Dis 2013; 15 (Suppl 2): 1–38.
Schlemper RJ, Riddell RH, Kato Y et al, The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47: 251–255.
Sobin LH, Gospodarowics MK, Wittekind C . TNM Classification of Malignant Tumours (ed 7). Wiley-Blackwell: West Sussex, UK, 2009.
Compton CC, Fielding LP, Burgart LJ et al, Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000; 124: 979–994.
Ueno H, Mochizuki H, Hashiguchi Y et al, Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004; 127: 385–394.
Dekker JW, Gooiker GA, Bastiaannet E et al, Cause of death the first year after curative colorectal cancer surgery; a prolonged impact of the surgery in elderly colorectal cancer patients. Eur J Surg Oncol 2014; 40: 1481–1487.
Dekker JW, van den Broek CB, Bastiaannet E et al, Importance of the first postoperative year in the prognosis of elderly colorectal cancer patients. Ann Surg Oncol 2011; 18: 1533–1539.
Verweij NM, Schiphorst AH, Maas HA et al, Colorectal Cancer Resections in the Oldest Old Between 2011 and 2012 in The Netherlands. Ann Surg Oncol 2016; 23: 1875–1882.
Komuta K, Batts K, Jessurun J et al, Interobserver variability in the pathological assessment of malignant colorectal polyps. Br J Surg 2004; 91: 1479–1484.
Volk EE, Goldblum JR, Petras RE et al, Management and outcome of patients with invasive carcinoma arising in colorectal polyps. Gastroenterology 1995; 109: 1801–1807.
Cooper HS, Deppisch LM, Gourley WK et al, Endoscopically removed malignant colorectal polyps: clinicopathologic correlations. Gastroenterology 1995; 108: 1657–1665.
Harris EI, Lewin DN, Wang HL et al, Lymphovascular invasion in colorectal cancer: an interobserver variability study. Am J Surg Pathol 2008; 32: 1816–1821.
Hansen TP, Fenger C, Kronborg O . The expression of p53, Ki-67 and urokinase plasminogen activator receptor in colorectal adenomas with true invasion and pseudoinvasion. APMIS 1999; 107: 689–694.
Mueller J, Mueller E, Arras E et al, Stromelysin-3 expression in early (pT1) carcinomas and pseudoinvasive lesions of the colorectum. Virchows Arch 1997; 430: 213–219.
Yantiss RK, Bosenberg MW, Antonioli DA et al, Utility of MMP-1, p53, E-cadherin, and collagen IV immunohistochemical stains in the differential diagnosis of adenomas with misplaced epithelium vs adenomas with invasive adenocarcinoma. Am J Surg Pathol 2002; 26: 206–215.
Panarelli NC, Somarathna T, Samowitz WS et al, Diagnostic Challenges Caused by Endoscopic Biopsy of Colonic Polyps: A Systematic Evaluation of Epithelial Misplacement With Review of Problematic Polyps From the Bowel Cancer Screening Program, United Kingdom. Am J Surg Pathol 2016; 40: 1075–1083.
Zhang D, Zhu H, Harpaz N . Overexpression of alpha1 chain of type XI collagen (COL11A1) aids in the diagnosis of invasive carcinoma in endoscopically removed malignant colorectal polyps. Pathol Res Pract 2016; 212: 545–548.
Dirschmid K, Kiesler J, Mathis G et al, Epithelial misplacement after biopsy of colorectal adenomas. Am J Surg Pathol 1993; 17: 1262–1265.
Acknowledgements
The authors thank the pathology departments of the participating hospitals for their cooperation in providing the hematoxylin-eosin stained slides. This investigator-initiated trial was supported by a grant of the Dutch Digestive Diseases Foundation (reference MG/2015-040).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Backes, Y., Moons, L., Novelli, M. et al. Diagnosis of T1 colorectal cancer in pedunculated polyps in daily clinical practice: a multicenter study. Mod Pathol 30, 104–112 (2017). https://doi.org/10.1038/modpathol.2016.165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.165
This article is cited by
-
Deep learning system for true- and pseudo-invasion in colorectal polyps
Scientific Reports (2024)