Abstract
EZH2, a member of the polycomb protein group, is an important methyltransferase that is overexpressed in various neoplasms. We found that in small cell B-cell lymphomas, EZH2 is expressed in <40% of neoplastic cells, with heterogenous signal intensity. In aggressive B-cell lymphomas, 70–100% of tumor cells were positive for EZH2 expression with high signal intensity, which correlated with a high proliferation rate. We investigated the potential signaling molecules that regulate EZH2 overexpression in aggressive B-cell lymphomas and found that 80% of cases of EZH2-positive diffuse large B-cell lymphoma show high p-ERK1/2 expression (average ~57% tumor cell positivity). In contrast, only a small percentage of tumor cells (~10%) show p-ERK1/2 expression in Burkitt lymphoma and double hit lymphoma. On average, 91 and 76% of neoplastic cells were positive for MYC expression in Burkitt lymphoma and double hit lymphoma, respectively, while only 20% neoplastic cells were positive for MYC expression in diffuse large B-cell lymphoma. None of the aggressive B-cell lymphomas showed significant p-STAT3 expression in EZH2-overexpressed cases. The correlation of EZH2 expression with aggressive behavior and proliferation rate in B-cell neoplasms suggests that this molecule may function as an oncogenic protein in these neoplasms, with possible regulation by different signaling cascades in different types of aggressive B-cell lymphomas: p-ERK-related signaling in diffuse large B-cell lymphoma, and MYC-related signaling in Burkitt lymphoma and double hit lymphoma. Furthermore, EZH2 and associated signaling cascades may serve as therapeutic targets for the treatment of aggressive B-cell lymphomas.
Similar content being viewed by others
Main
EZH2 is an important enzymatic subunit of the epigenetic regulator polycomb repressive complex 2 (PRC2) and functions as a methytransferase that targets the lysine 27 of histone H3 and controls gene silencing through posttranslational modification.1 EZH2 is frequently overexpressed in various non-hematological malignancies and functions as an oncogenic protein for tumor cell proliferation, metastasis, and survival.2 A number of intracytoplasmic oncogenic signaling molecules, transcription factors, and tumor-suppressor miRNAs regulate EZH2 expression in these non-hematological neoplasms. In the triple-negative and ERBB2-overexpressing aggressive subtypes of breast cancer, studies using promoter analysis and in vitro inhibitor methods demonstrated that the MEK/ERK/Elk-1 signaling pathway leads to EZH2 overexpression.3 In a subset of invasive breast carcinomas and moderately to poorly differentiated non-small cell lung cancers, AKT(Ser473) phosphorylation was closely associated with EZH2 overexpression.4, 5 In prostate and hepatocellular carcinomas, upregulation of MYC protein results in the overexpression of EZH2 through reduction of miR-26a, miR26b, and miR-101. In addition, MYC can directly bind to and activate the EZH2 promoter.6, 7, 8 In epithelial ovarian cancer, NF-YA, the regulatory subunit of CCAAT-binding transcription factor, is highly expressed and upregulates EZH2 at the transcriptional level.9 In colorectal carcinoma, STAT3 is constitutively activated, directly binds to the EZH2 promoter, and regulates its expression, which is thought to contribute to colon cancer development and metastasis.10,11,12,13
Recent evidence supports a more complicated mechanism of EZH2's contribution to tumor development in hematopoietic malignancies. Hyperactivation of polycomb group proteins, particularly EZH2, has been linked to the pathogenesis of a subset of hematological malignancies, including some B- and T-cell lymphomas as well as myeloid disorders.14, 15, 16 For example, in a subset of follicular lymphomas and germinal center subtype of diffuse large B-cell lymphomas, a change of amino-acid tyrosine 641 (Y641) has been identified as a recurrent somatic mutation in the EZH2 gene, leading to increased enzymatic activity.17 The oncogenic role of the Y641 mutation was further confirmed in an engineered mouse model in which conditional expression of mutant EZH2 in germinal center B cells induced germinal center hyperplasia and promoted lymphomagenesis in cooperation with BCL2 overexpression.18 In addition to the Y641 mutation, A687V and A677G mutations have been identified as gain-of-function mutations of EZH2 in B-cell lymphomas.19, 20 Interestingly, there is also evidence that loss-of-function mutations and deletions of EZH2 and associated genes leads to the development of mouse and human T-acute lymphoblastic leukemia, suggesting that EZH2 functions as a tumor-suppressor gene for this hematopoietic neoplasm.21, 22
Because of the complicated role of EZH2 in the development of non-hematological as well as hematological malignancies, we investigated the expression of EZH2 in a range of small cell and aggressive B-cell non-Hodgkin lymphomas. We found that EZH2 expression correlates with aggressive behavior and with the expression of specific signaling molecules in aggressive B-cell lymphomas that are known to cause EZH2 overexpression and contribute to oncogenesis.
Methods and materials
Case Selection
Lymphoma cases were obtained from the files of the Department of Pathology, Brigham and Women’s Hospital, Boston, MA, from 1990 to 2015, with the institutional internal review board’s approval. A range of small cell and aggressive B-cell non-Hodgkin lymphomas were included in the study, including 12 cases of multiple myeloma, 12 cases of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia, 18 cases of chronic lymphocytic leukemia/small lymphocytic lymphoma, 9 cases of mantle cell lymphoma, 21 cases of marginal zone lymphoma, 10 cases of follicular lymphoma grades 1–2/3, 14 cases of hairy cell leukemia, 10 cases of hairy cell leukemia variant, 33 cases of diffuse large B-cell lymphoma, 19 cases Burkitt lymphoma, 5 cases of Richter transformation of chronic lymphocytic leukemia/small lymphocytic lymphoma, 6 cases of follicular lymphoma grade 3/3, and 22 cases of double hit lymphoma (corresponding to the World Health Organization diagnosis of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma (9 cases) and the World Health Organization diagnosis of diffuse large B-cell lymphoma (13 cases), and including 13 cases with MYC+BCL2 translocations and 9 cases with MYC+BCL6 translocations), 19 cases of primary mediastinal large B-cell lymphoma, and 11 cases of B lymphoblastic leukemia/lymphoma. The pathological diagnoses were established according to the criteria of the 2008 World Health Organization classification, based on morphological, immunohistochemical (IHC), cytogenetics, and molecular findings. Historical data were used for fluorescence in situ hybridization studies. Diagnostic slides from all cases were reviewed by two hematopathologists (XT and DMD).
Immunohistochemistry
Immunostaining for EZH2 (Cell Signaling 5246), p-STAT3 (Cell Signaling 9145), and MYC (Abcam ab32072) were performed on the Leica Bond automated stainer, using the Bond Polymer Refine Detection Kit (cat. no. DS9800).23 Heat-induced epitope retrieval was completed using Bond Epitope Retrieval Solution 2 (cat. no. AR9640), an EDTA-based pH 9.0 solution, for 20 min at 100 °C. Primary antibodies were applied at the optimized dilution (1:100) for 30 min at ambient temperature. For p-ERK1/2 (Cell Signaling 4370) and Ki-67 (Abcam ab16667) immunostaining, heat-induced epitope retrieval was completed using Bond Epitope Retrieval Solution 1 (cat. no. AR9961), a citrate-based pH 6.0 solution, for 30 min at 100 °C. Primary antibody was applied at the optimized dilution (1:150) for 30 min at ambient temperature. This was followed by a 10-min application of the post primary, 10 min of polymer, and 5 min of peroxide block. Staining was visualized with a 3,3'-diaminobenzidine chromogen for 10 min and counterstained with hematoxylin. The slides were then removed from the autostainer, rinsed in running tap water, dehydrated through alcohols and xylene, and coverslipped.
The cases were scored for the percentage of positive tumor cells (0–100%) and for staining intensity (0–3) by two hematopathologists (XT and DMD). Reactivity for EZH2, p-ERK1/2, MYC, and p-STAT3 was considered as positive if there was more than focal staining (5%) of neoplastic cells. EZH2 staining was considered homogeneous if ≥60% of the neoplastic cells exhibited staining with 2+ or 3+ intensity. P-ERK1/2, MYC, and p-STAT3 staining was considered positive if ≥5% of neoplastic cells were positive, as described previously.24 Statistical analysis was performed using Graphpad Prism (Graphpad Software, La Jolla, CA, USA).
Results
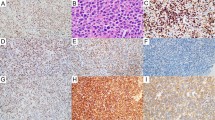
To investigate the tumorigenic role of EZH2 in B-cell lymphomas, IHC staining for EZH2 was first performed on small cell and aggressive B-cell lymphomas. In the 106 cases of small cell B-cell lymphomas studied, 86% of cases were positive for EZH2, with 5–40% of neoplastic cells positive for EZH2, depending on lymphoma subtype, with heterogeneous nuclear staining (variable positive intensity of staining from 1+ to 3+; Table 1 and Figure 1). In the 115 cases of aggressive B-cell lymphomas studied, including those transformed from small cell B-cell lymphomas, 97% of cases were positive for EZH2, with 70–100% of tumor cells positive for EZH2, depending on lymphoma subtype, all of which showed strong homogenous nuclear staining for EZH2 (positive intensity of 3+, Table 1 and Figure 1), a significant difference in EZH2 expression compared with small cell B-cell lymphomas (P<0.01). These data show that EZH2 expression correlates with aggressive behavior in B-cell neoplasms. The high level of EZH2 expression in aggressive B-cell lymphomas suggests that this molecule may function as an oncogenic protein in these neoplasms, similar to its role in the non-hematological aggressive solid tumors such as breast, colorectal, prostate, and hepatocellular carcinomas.
EZH2 expression varied among the small cell B-cell lymphomas. In hairy cell leukemia, a relatively indolent B-cell neoplasm, 5–10% of tumor cells expressed EZH2 with variable intensity, while in hairy cell leukemia variant, a more aggressive B-cell neoplasm with shorter median survival,25 30–40% of neoplastic cells expressed EZH2, with stronger intensity (P<0.01, Figure 2). EZH2 expression was higher in mantle cell lymphoma, with positivity in 30% of neoplastic cells on average, compared with other types of small cell B-cell lymphomas (except hairy cell leukemia variant), which had an average of 12% of neoplastic cell's positive for EZH2 (P<0.01; Table 1). These results show that EZH2 expression correlates with aggressiveness in small cell B-cell lymphomas.
EZH2 has been shown to be required for tumor cell proliferation and is associated with a high proliferation index in many types of non-hematological cancer types, including breast, prostate, endometrium, and melanoma.26 We examined the proliferation index in the small cell and aggressive B-cell lymphomas by assessing Ki-67 nuclear staining and correlated the results with EZH2 expression. We found that the Ki-67 proliferation index in small cell B-cell lymphomas ranged from 5 to 35%, while in aggressive B-cell lymphomas Ki-67 ranged from 70 to 100% of the neoplastic cells (Table 1). These data show that EZH2 overexpression correlates with proliferation index in B-cell lymphomas (R2=0.9249; Figure 1).
A number of signaling cascade-associated molecules, including p-ERK1/2, MYC, and p-STAT3, have been shown to upregulate EZH2 expression in non-hematological neoplasms, including breast, prostate, and colorectal cancers. To explore potential mechanisms of EZH2 overexpression in aggressive B-cell lymphomas, p-ERK1/2, MYC, and p-STAT3 expression was studied by IHC in diffuse large B-cell lymphoma, double hit lymphoma, and Burkitt lymphoma. We found that 40–80% of neoplastic cells in diffuse large B-cell lymphoma (mean 57%±17%, 24/30 cases) were positive for p-ERK expression, but <30% of neoplastic cells were positive for p-ERK expression in Burkitt lymphoma (mean 13%±10%, 15 cases) and double hit lymphoma (mean 10%±9%, 17 cases). In contrast, 80–100% and 50–90% of neoplastic cells were positive for MYC expression in Burkitt lymphoma (mean 91%±7%, 19 cases) and double hit lymphoma (mean 76%±14%, 29 cases), respectively, but only 5–50% of neoplastic cells were positive for MYC expression in diffuse large B-cell lymphoma (mean 20%±17%, 30 cases). There were significant differences in both MYC and p-ERK expression in Burkitt lymphoma and double hit lymphoma versus diffuse large B-cell lymphoma (P<0.01), but no significant differences in double hit lymphoma cases with MYC+BCL2 translocations compared with double hit lymphoma cases with MYC+BCL6 translocations or in double hit lymphoma cases initially classified as B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma compared with double hit lymphoma cases initially classified as diffuse large B-cell lymphoma. None of the aggressive B-cell lymphomas showed significant p-STAT3 positivity in neoplastic cells (<10% cell positivity with heterogeneous intensity; Table 2, Figure 3).
Discussion
Aberrant epigenetic regulation has been shown to have a central role in the pathogenesis of many non-hematological and hematological malignancies. The maintenance of appropriate DNA methylation is an important epigenetic modification process in silencing specific gene transcription involved in critical biological processes, including cellular development and stem cell plasticity.27, 28, 29, 30 Disruption of methylation profiles and loss of epigenetic stability is critical in malignant transformation. EZH2 functions as an important histone methyltransferase to regulate DNA methylation and control gene expression. Over the past decade, studies have established that EZH2 is overexpressed in many non-hematological solid tumor types and its high expression is associated with tumor aggressiveness and progression.31, 32 Furthermore, disruption of the PRC2/EZH2 complex either pharmacologically or genetically can block proliferation of EZH2-overexpressing cancer cell lines and neoplasms in mouse models of EZH2 overexpression.33, 34 These findings support an oncogenic role for EZH2 in non-hematological tumor development.
However, frequent homozygous and heterozygous EZH2 deletions or inactivating mutations have been found in myeloid neoplasms35, 36, 37, 38 and are predictive of poor survival.39, 40 In addition, loss of Ezh2 in mouse hematopoietic stem cells is sufficient to cause aggressive T-acute lymphoblastic leukemia.21, 22 These studies demonstrate that the loss rather than overexpression of EZH2 may also contribute to tumorigenesis in myeloid and some lymphoid neoplasms, presumably through derepression of oncogenes owing to lack of H3K27 methylation.
B-cell non-Hodgkin lymphomas are mature B-cell neoplasms with a wide spectrum of morphological and immunophenotypic features and varying clinical aggressiveness. In this study, we examined EZH2 expression in a range of small cell and aggressive B-cell lymphomas and found that EZH2 expression correlates with aggressive behavior in B-cell neoplasms, with high levels of EZH2 expression seen in aggressive B-cell lymphomas. Furthermore, among the small cell B-cell lymphomas, EZH2 expression correlates with more aggressive clinical behavior. For example, we found that EZH2 expression is significantly higher in hairy cell leukemia variant, a more aggressive B-cell neoplasm with shorter median survival, than in classical hairy cell leukemia (P<0.01). Similarly, mantle cell lymphoma exhibits high EZH2 expression compared with other types of small cell B-cell lymphomas and has a more aggressive clinical course. Proliferation rate typically correlates with tumor grade in B-cell non-Hodgkin lymphomas, and we found that EZH2 expression correlates with proliferation rate, as assessed by Ki-67 IHC staining, in B-cell non-Hodgkin lymphomas, as has been previously reported in a range of human cancers. These results suggest an oncogenic role for EZH2 in B-cell neoplasms, similar to its role in non-hematological solid tumors.
The mechanism of EZH2 overexpression has been extensively studied in non-hematological cancers. One of the prototypic oncogenic signaling pathways, the RAS/MEK/ERK/ELK pathway, which is upregulated in many cancer cells, is linked to the overexpression of EZH2 in triple-negative and ERBB2-overexpressing subtypes of breast cancer.2 MEK inhibitor and Elk-1 siRNA successfully blocked EZH2 mRNA and protein expression levels in cells from aggressive breast cancers,3 suggesting that the ERK signaling cascade is upstream of EZH2 and regulates its expression. The role of the RAS/MEK/ERK/ELK pathway in the regulation of EZH2 in lymphoid neoplasms is unclear. Here we focused on three subtypes of high-grade B-cell lymphoma with EZH2 overexpression, diffuse large B-cell lymphoma, double hit lymphoma, and Burkitt lymphoma, to understand the potential role of the ERK pathway in EZH2 expression. We found that 80% of diffuse large B-cell lymphoma cases are positive for p-ERK1/2 (57%±17% mean tumor cell staining), while cases of double hit lymphoma and Burkitt lymphoma show weak to negative p-ERK1/2 staining (13±10% and 10±9% mean tumor cell staining, respectively; P<0.01). These results suggest that the RAS/MEK/ERK/ELK pathway may preferentially upregulate EZH2 expression in diffuse large B-cell lymphoma, compared with Burkitt lymphoma and double hit lymphoma.
MYC is an important transcription factor, cell cycle regulator, and oncogenic protein in tumorigenesis that causes EZH2 overexpression in solid tumors, including prostate carcinoma and hepatocellular carcinoma.6, 7, 8 We found all the cases of Burkitt lymphoma and double hit lymphoma show high MYC expression (80–100 and 50–90% tumor cells are positive, respectively), which correlates with high EZH2 expression in these neoplasms; however, only 20% of tumor cells in the diffuse large B-cell lymphoma cases show positive MYC staining. These results suggest that MYC may preferentially upregulate EZH2 expression in Burkitt lymphoma and double hit lymphoma, compared with diffuse large B-cell lymphoma. We also studied p-STAT3, a regulator of MYC activation that has an important role in cancer development. P-STAT3 has been show to upregulate EZH2 expression in colorectal cancer.12, 13 We found that none of the aggressive B-cell lymphomas studied showed significant p-STAT3 positivity in neoplastic cells (<10% cell positivity), suggesting that it may not have a significant role in EZH2 overexpression in aggressive B-cell lymphomas.
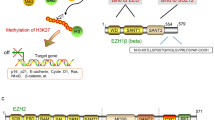
Our findings suggest that different signaling pathways and molecules may be involved in directing EZH2 overexpression in different types of aggressive B-cell lymphomas (Figure 4). In diffuse large B-cell lymphomas, the RAS/MEK/ERK pathway may have an important role in the regulation of EZH2 high expression, while in double hit lymphoma and Burkitt lymphoma, MYC expression, and hence MYC-related signaling, appears to be associated with EZH2 overexpression. P-STAT3 is not co-expressed with EZH2 in aggressive B-cell lymphomas, so it does not appear to have a role in the overexpression of EZH2 in aggressive B-cell lymphomas (Figure 4). Additional, in vitro studies are needed to further investigate the role of ERK1/2 and MYC signaling in EZH2 overexpression in aggressive B-cell lymphomas.
A proposed model for the regulation of EZH2 expression in different types of aggressive B-cell lymphomas by differential signaling cascades, based on the observation that p-ERK1/2 expression, but not MYC expression, correlates with high EZH2 expression in diffuse large B-cell lymphoma and high MYC expression, but not p-ERK1/2 expression, is associated with increased EZH2 expression in Burkitt lymphoma and double hit lymphoma.
EZH2 has been shown to have a multi-faceted role in cancer progression, functioning as an oncogenic protein through overexpression in solid non-hematological tumors and as a tumor-suppressor gene in myeloid and some lymphoid neoplasms. Because of its complex role in cancer development, attempts to therapeutically target EZH2 and EZH2-mediated signaling are emerging as an important strategy for cancer treatment. Several inhibitors of enzymes controlling epigenetic modifications, such as DNA methyltransferases and histone deacetylases, have shown promising antitumor effects.41, 42 Recently, a small-molecule inhibitor of EZH2 has been developed that selectively blocks EZH2 methyltransferase activity and reduces global H3K27 methylation.43 An EZH2 inhibitor has been used in early clinical trials in patients with advanced solid tumors or relapsed/refractory diffuse large B-cell lymphoma and follicular lymphoma (Clinical trails.gov, ID:NCT01897571). Our findings suggest additional B-cell lymphomas that may be suitable targets for EZH2 inhibitor treatment. In addition, the targeting of specific signaling pathways that upregulate EZH2 expression is another potential strategy for the treatment of aggressive B-cell lymphomas.
References
Simon JA, Kingston RE . Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol 2009;10:697–708.
Fujii S, Ito K, Ito Y et al. Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by increasing histone H3 methylation. J Biol Chem 2008;283:17324–17332.
Fujii S, Tokita K, Wada N et al. MEK–ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes. Oncogene 2011;30:4118–4128.
Geng J, Li X, Zhou Z et al. EZH2 promotes tumor progression via regulating VEGF-A/AKT signaling in non-small cell lung cancer. Cancer Lett 2015;359:275–287.
Gonzalez M, DuPrie M, Krueger H et al. Histone methyltransferase EZH2 induces Akt-dependent genomic instability and BRCA1 inhibition in breast cancer. Cancer Res 2001;71:2360–2370.
Koh CM, Iwata T, Zheng Q et al. Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms. Oncotarget 2011;2:669–683.
Wang L, Zhang X, Jia LT et al. c-Myc mediated epigenetic silencing of MicroRNA-101 contributes to dysregulation of multiple pathways in hepatocellular carcinoma. Hepatology 2014;59:1850–1863.
Sander S, Bullinger L, Klapproth K et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood 2008;112:4202–4212.
Garipov A, Li H, Bitler BG et al. NF-YA underlies EZH2 upregulation and is essential for proliferation of human epithelial ovarian cancer cells. Mol Cancer Res 2013;11:360–369.
Spano JP, Milano G, Rixe C et al. JAK/STAT signalling pathway in colorectal cancer: a new biological target with therapeutic implications. Eur J Cancer 2006;42:2668–2670.
Lassmann S, Schuster I, Walch A et al. STAT3 mRNA and protein expression in colorectal cancer: effects on STAT3-inducible targets linked to cell survival and proliferation. J Clin Pathol 2007;60:173–179.
Xiong H, Zhang ZG, Tian XQ et al. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia 2008;10:287–297.
Lin YW, Ren LL, Xiong H et al. Role of STAT3 and vitamin D receptor in EZH2-mediated invasion of human colorectal cancer. J Pathol 2013;230:277–290.
Shi M, Shahsafaei A, Liu C et al. Enhancer of zeste homolog 2 is widely expressed in T-cell neoplasms, is associated with high proliferation rate and correlates with MYC and p-STAT3 expression in a subset of cases. Leuk Lymphoma 2015;56:2087–2091.
Visser HP, Gunster MJ, Kluin-Nelemans HC et al. The Polycomb group protein EZH2 is upregulated in proliferating, cultured human mantle cell lymphoma. Br J Haematol 2001;112:950–958.
Ernst T, Chase AJ, Score J et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet 2010;42:722–726.
Morin RD, Johnson NA, Severson TM et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 2010;42:181–185.
Beguelin W, Popovic R, Teater M et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 2013;23:677–692.
Majer CR, Jin L, Scott MP et al. A687V EZH2 is a gain-of-function mutation found in lymphoma patients. FEBS Lett 2012;586:3448–3451.
McCabe MT, Graves AP, Ganji G et al. Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27). Proc Natl Acad Sci USA 2012;109:2989–2994.
Ntziachristos P, Tsirigos A, Vlierberghe P . Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat Med 2012;18:298–301.
Simon C, Chagraoui J, Krosl J et al. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes Dev 2012;26:651–656.
Dorfman DM, Greisman HA, Shahsafaei A . Loss of expression of the WNT/beta-catenin-signaling pathway transcription factors lymphoid enhancer factor-1 (LEF-1) and T cell factor-1 (TCF-1) in a subset of peripheral T cell lymphomas. Am J Pathol 2003;162:1539–1544.
Kluk MJ, Chapuy B, Sinha P et al. Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One 2012;7:e33813.
Robak T . Management of hairy cell leukemia variant. Leuk Lymphoma 2011;52 (Suppl 2):53–56.
Bracken AP, Pasini D, Capra M et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 2003;22:5323–5335.
Mardis ER, Ding L, Dooling DJ et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 2009;361:1058–1066.
Bejar R, Stevenson K, Abdel-Wahab O et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med 2011;364:2496–2506.
Patel JP, Gonen M, Figueroa ME et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012;366:1079–1089.
Haferlach T, Nagata Y, Grossmann V et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2014;28:241–247.
Varambally S, Dhanasekaran SM, Zhou M et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002;419:624–629.
Bracken AP, Pasini D, Capra M et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 2003;22:5323–5335.
Tan J, Yang X, Zhuang L et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev 2007;21:1050–1063.
Wilson BG, Wang X, Shen X et al. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 2010;18:316–328.
Ernst T, Chase AJ, Score J et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet 2010;42:722–726.
Makishima H, Jankowska AM, Tiu RV et al. Novel homo- and hemizygous mutations in EZH2 in myeloid malignancies. Leukemia 2010;24:1799–1804.
Nikoloski G, Langemeijer SM, Kuiper RP et al. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet 2010;42:665–667.
Score J, Hidalgo-Curtis C, Jones AV et al. Inactivation of polycomb repressive complex 2 components in myeloproliferative and myelodysplastic/myeloproliferative neoplasms. Blood 2012;119:1208–1213.
Bejar R, Stevenson K, Abdel-Wahab O et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med 2011;364:2496–2506.
Guglielmelli P, Biamonte F, Score J et al. EZH2 mutational status predicts poor survival in myelofibrosis. Blood 2011;118:5227–5234.
Egger G, Liang G, Aparicio A et al. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004;429:457–463.
Wee S, Dhanak D, Li H et al. Targeting epigenetic regulators for cancer therapy. Ann NY Acad Sci 2014;1309:30–36.
Garapaty-Rao S, Nasveschuk C, Gagnon A et al. Identification of EZH2 and EZH1 small molecule inhibitors with selective impact on diffuse large B-cell lymphoma cell growth. Chem Biol 2013;20:1329–1339.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tian, X., Pelton, A., Shahsafaei, A. et al. Differential expression of enhancer of zeste homolog 2 (EZH2) protein in small cell and aggressive B-cell non-Hodgkin lymphomas and differential regulation of EZH2 expression by p-ERK1/2 and MYC in aggressive B-cell lymphomas. Mod Pathol 29, 1050–1057 (2016). https://doi.org/10.1038/modpathol.2016.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.114
This article is cited by
-
EZH2 expression is associated with inferior overall survival in mantle cell lymphoma
Modern Pathology (2021)
-
Subcellular localization of EZH2 phosphorylated at T367 stratifies metaplastic breast carcinoma subtypes
Breast Cancer (2021)
-
Physiological levels of the PTEN-PI3K-AKT axis activity are required for maintenance of Burkitt lymphoma
Leukemia (2020)
-
Expression of enhancer of zeste homolog 2 (EZH2) protein in histiocytic and dendritic cell neoplasms with evidence for p-ERK1/2-related, but not MYC- or p-STAT3-related cell signaling
Modern Pathology (2018)