Abstract
Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for patients with malignant peritoneal mesothelioma has resulted in improved disease control and increased survival. Despite these results, there are significant perioperative risks associated with this aggressive procedure that necessitate consideration of prognostic markers during patient selection. The molecular pathogenesis of peritoneal mesothelioma remains relatively unknown, but extrapolation of findings from their pleural counterpart would suggest frequent alterations in CDKN2A, NF2, and BAP1. Homozygous deletions in CDKN2A portend a worse overall survival in peritoneal mesothelioma. However, the prevalence and prognostic significance of NF2 and BAP1 abnormalities has not been studied. Dual-color fluorescence in situ hybridization using CDKN2A and NF2 locus-specific probes and BAP1 immunohistochemistry identified homozygous CDKN2A deletions (n=25, 29%), hemizygous NF2 loss (n=30, 35%), and/or loss of BAP1 protein expression (n=49, 57%) in 68 of 86 (79%) peritoneal mesotheliomas. Homozygous CDKN2A deletions or hemizygous NF2 loss correlated with shorter progression-free survival (P<0.02) and poor overall survival (P<0.03). Moreover, the significance of these findings was cumulative. Patients harboring both homozygous CDKN2A deletions and hemizygous NF2 loss had a 2-year progression-free survival rate of 9% with a median of 6 months (P<0.01) and overall survival rate of 18% with a median of 8 months (P<0.01). By multivariate analysis, combined homozygous CDKN2A deletions and hemizygous NF2 loss was a negative prognostic factor for both progression-free survival and overall survival, independent of patient age, peritoneal cancer index, completeness of cytoreduction, and extent of invasion. In contrast, loss of BAP1 was not associated with clinical outcome. In summary, homozygous deletions in CDKN2A and hemizygous loss of NF2 as detected by fluorescence in situ hybridization would confer a poor clinical outcome and may guide future treatment decisions for patients with peritoneal mesothelioma.
Similar content being viewed by others
Main
Malignant mesothelioma is a rare, but aggressive neoplasm, which arises from the mesothelial lining of the pleura, peritoneum, pericardium, and tunica vaginalis. Although the majority of mesotheliomas are pleural in origin, 10–15% of cases arise from the peritoneum.1, 2 In the United States, peritoneal mesothelioma has an incidence of 400 occurrences per year.3 Patients commonly present with vague and nonspecific symptoms including abdominal distension, pain, and weight loss.4 Consequently, patients with peritoneal mesothelioma are often diagnosed late in their disease course and prognosis is dismal with a median overall survival (OS) of 10–12 months.5, 6
To date, a curative therapeutic option for patients with peritoneal mesothelioma is lacking. Considering that peritoneal mesotheliomas are typically localized to the abdominal cavity and only a few cases of intra- or extra-abdominal invasion have been reported, treatment strategies have aimed at surgical debulking and controlling disease progression within the peritoneal cavity. Currently, cytoreductive surgery (CRS), combined with hyperthermic intraperitoneal chemoperfusion (HIPEC), has emerged as the standard treatment for patients with peritoneal mesothelioma.7 Clinical trials have shown that treatment with CRS and HIPEC demonstrates improved median OS ranging from 27 to 46 months.8, 9, 10, 11, 12 Despite these favorable results, CRS and HIPEC therapy is associated with significant perioperative morbidity and mortality.10, 13, 14, 15 Thus, patient selection is critical to maximize clinical outcome and to exclude patients who will not benefit from a potentially life-threatening procedure.
Although little is known with regards to the pathogenesis and key genetic abnormalities of peritoneal mesothelioma, their pleural counterpart have been the subject of comparative genomic hybridization, candidate gene-sequencing approaches and whole-exome sequencing.16, 17, 18, 19, 20 Integrative analysis of mutations and somatic copy-number alterations has revealed frequent inactivation in CDKN2A, NF2, and BAP1. Moreover, the status of these three genes has significant prognostic implications. Homozygous deletions in CDKN2A are the most frequent genetic alteration in pleural mesothelioma with a reported deletion rate ranging from 60 to 74% by fluorescence in situ hybridization.21, 22, 23 In addition, homozygous CDKN2A deletions are a poor prognostic indicator for patients with pleural mesothelioma.22, 24, 25 NF2, located on chromosome 22q12, is mutated in 50% of pleural mesotheliomas with corresponding loss of the wild-type allele by deletion of either 22q or all of chromosome 22.16, 26 Hemizygous loss of NF2 is associated with increased mesothelioma proliferation, invasiveness, spreading, and migration.26, 27, 28
Mutations in the nuclear deubiquitinase, BAP1, result in either complete absence of protein expression or cytoplasmic sequestration of BAP1, which can be detected by immunohistochemistry, in 27–67% of pleural mesotheliomas.18, 29, 30 In contrast to CDKN2A and NF2 alterations, loss of BAP1 protein expression portends improved prognosis for patients with pleural mesothelioma.30, 31
Analogous to pleural mesotheliomas, homozygous CDKN2A deletions are also present in peritoneal mesotheliomas and confer an unfavorable outcome after CRS and HIPEC.32 However, the prevalence and prognostic significance of NF2 and BAP1 alterations in peritoneal mesotheliomas remains relatively unknown. We, therefore, evaluated the status of CDKN2A, NF2, and BAP1 within a large cohort of peritoneal mesotheliomas. These findings were correlated with various clinicopathologic features including progression-free survival (PFS) and OS.
Materials and methods
Malignant Peritoneal Mesothelioma Study Cohort and Tissue Microarray Construction
Study approval was obtained from the University of Pittsburgh institutional review board (IRB# PRO14070080). Between 2001 and 2014, all patients diagnosed with malignant peritoneal mesothelioma and underwent CRS with HIPEC at the University of Pittsburgh Medical Center were identified. Well-differentiated peritoneal mesotheliomas were specifically excluded from this study. In total, 86 patients had archival formalin-fixed, paraffin-embedded (FFPE) tissue blocks available for ancillary studies. Corresponding hematoxylin and eosin-stained slides and associated immunohistochemical stains (e.g., calretinin, WT-1, D2-40, and CK5/6) were reviewed to confirm the pathologic diagnosis of peritoneal mesothelioma. Each case was classified into three histologic subtypes that include epithelioid, biphasic, and sarcomatoid. Classification of either epithelioid or sarcomatoid mesothelioma required at least 90% of the tumor to be composed of this morphologic pattern. Biphasic mesothelioma required both components to represent at least 10% of the tumor. The extent of invasion from the peritoneal surface was scored as either limited to the underlying adipose tissue or into the organ viscera. The presence of lymph nodes and mesothelioma involvement was recorded.
The clinical and intraoperative reports were also reviewed to document patient gender, age, asbestos exposure, peritoneal cancer index (PCI) and completeness of cytoreduction (CC) score. The PCI was determined at the time of surgical exploration and represents quantification and distribution of disease within the peritoneal cavity. The index is based on tumor extent in 13 separate regions that include the central (periumbilical) abdomen, right upper abdomen, epigastrium, left upper abdomen, left flank, left lower abdomen, pelvis, right lower abdomen, right flank, upper jejunum, lower jejunum, upper ileum, and lower ileum.33, 34 A score for each region is allocated by measuring the maximum thickness of the largest tumor nodule (no tumor=0; <0.5 cm=1; 0.5 to 5 cm=2; and >5 cm or confluent tumors=3). The PCI has a maximum score of 39. CC scores were performed at the end of surgical resection and measure the extent of residual disease. CC scores were defined as follows: CC-0=no visible residual disease; CC-1=residual tumor<0.25 cm; CC-2=residual tumor 0.25 cm to 2.5 cm; and CC-3=residual tumor>2.5 cm.11
High-density tissue microarrays were constructed using archival FFPE tissue blocks. Three, 1.0 mm-sized cores were punched from representative areas of each patient’s tumor and collected into recipient blocks. In addition, whole sections from 12 of the 86 peritoneal mesotheliomas were randomly selected to confirm the adequacy of tissue microarrays for subsequent analysis by fluorescence in situ hybridization and immunohistochemistry.
Fluorescence In Situ Hybridization
Dual-color fluorescence in situ hybridization was performed for both CDKN2A and NF2, as previously reported.22, 23, 32 CDKN2A was assessed using a Spectrum-Orange labeled, locus-specific probe (Abbott Molecular, Des Plains, IL, USA) with a Spectrum Green-labeled chromosome 9 centromeric (CEP9) probe.22 Probes for NF2 assessment included a FITC-labeled chromosome 22 centromeric (CEP22q) probe and a Texas Red-labeled, locus-specific NF2 probe (Abnova, Walnut, CA, USA). Staining of tissue microarrays and whole sections were performed as previously described using 4-μm unstained paraffin sections.22, 23 Each core on the tissue microarrays was identified and only individual and well-delineated cells were scored; overlapping cells were excluded from the analysis. For both tissue microarrays and whole sections, at least 60 cells were scored for each case and control. Each tumor was assessed by the average and the maximum numbers of copies of the either CDKN2A or NF2 per cell and the average ratio of the gene to CEP9 and CEP22q copy numbers, respectively.22, 23, 35
Immunohistochemistry
Immunohistochemical labeling was performed on 4-μm unstained paraffin sections for both the tissue microarrays and whole sections. Slides were deparaffinized with serial xylene treatments and subjected to antigen retrieval using heated citrate solution (pH 9.0) at 100 oC for 10 min. Immunolabeling for BAP1 (C-4 mouse monoclonal, dilution 1:100, Santa Cruz, CA, USA) was performed on the automated Ventana Benchmark XT system using the biotin-free Ventana OptiView DAB IHC Detection Kit (Ventana Medical Systems, Tucson, AZ, USA). Immunohistochemical scoring of BAP1 expression was performed similar to those published previously.17, 29 Assessment of BAP1 was done blinded to any other patient data including outcome. Intact or “positive” expression of BAP1 was defined as nuclear staining within tumor cells, using stromal cells as a positive internal control. Loss or “negative” staining was scored in cases where the tumor lacked nuclear immunolabeling. Similarly, representative whole sections were also stained to confirm loss of BAP1 nuclear expression and assess for intratumoral heterogeneity. Intratumoral heterogeneity of BAP1 staining was not observed.
Statistical Analysis
To compare the categorical data χ2 analysis or Fisher exact tests were used, and analysis of variance was used to compare the continuous variables. Survival curves were constructed using the Kaplan–Meier method and differences between groups were evaluated by the log-rank test. PFS was calculated from the date of surgery to the date of recurrence or censoring. OS was calculated as the time from the date of surgery to the date of death or censoring. The prognostic significance of clinical and pathologic characteristics was determined using univariate Cox regression analysis. Multivariate analyses of significant risk factors by univariate analysis were performed using Cox proportional hazard regression to identify independent risk factors for both PFS and OS. All statistical analyses were performed using the SPSS Statistical software, version 22 (IBM, Armonk, NY, USA) and statistical significance was defined as a P-value of <0.05.
Results
Clinical and Pathologic Characteristics
The clinical and pathologic features of the peritoneal mesothelioma study cohort are summarized in Table 1. Patients at diagnosis ranged in age from 19 to 83 years (mean, 53.6 years; median, 54 years) and were predominantly male (60 of 86, 70%) with a male-to-female ratio of 2.3 to 1. Past medical history was available for 72 (84%) patients with asbestos exposure documented in 18 (25%) cases. None of the patients reported a family history of peritoneal or pleural mesothelioma. Seventeen of 86 (20%) of patients were documented to have received preoperative chemotherapy. All 86 patients underwent CRS with HIPEC. Tumor burden was calculated at the time of surgery using PCI, which ranged from 8 to 39 (mean, 23; median, 24). The CC was scored for 83 (97%) cases and consisted of the following: 37 patients were CC-0, 30 patients were CC-1, 7 patients were CC-2, and 9 patients were CC-3. Microscopically, the predominant histologic subtype among the peritoneal mesotheliomas was epithelioid (75 of 86, 87%). Of the remaining morphologic patterns, 2 (2%) were sarcomatoid and 9 (11%) were biphasic. The extent of mesothelioma invasion from the serosal surface was limited to the surrounding fat for 46 (53%) cases and into the visceral parenchyma for 40 (47%) cases. Lymph nodes were submitted for pathologic review in 48 cases with 14 (29%) harboring metastases.
CDKN2A and NF2 Fluorescence In Situ Hybridization
CDKN2A deletions were detected in 42 of 86 (49%) peritoneal mesotheliomas. Twenty-five (29%) cases harbored a homozygous deletion in CDKN2A (Figures 1a and b), and 17 (20%) cases had monosomy at chromosome 9. Excluding tumors with chromosome 9 monosomy, hemizygous CDKN2A deletions were not seen. NF2 deletions were identified in 30 of 86 (35%) mesotheliomas and characterized by hemizygous loss. Hemizygous NF2 deletions occurred in 4 (5%) cases, and chromosome 22 monosomy in 26 (30%) cases (Figures 1c and d). Homozygous deletions in NF2 were not observed. In total, 34 (40%) peritoneal mesotheliomas had either a homozygous CDKN2A deletion or hemizygous NF2 loss, and 11 (13%) tumors had both homozygous CDKN2A deletions and hemizygous NF2 loss.
Dual-color fluorescence in situ hybridization using locus-specific probes for CDKN2A (orange) and NF2 (red) and chromosome 9 centromeric and chromosome 22 centromeric probes (green), respectively. Representative examples of peritoneal mesotheliomas with a normal copy number for CDKN2A (a) and NF2 (c). In contrast, homozygous deletions in CDKN2A were characterized by loss of both orange signals, but retention of at least one green signal (b). Hemizygous NF2 loss consisted of either monosomy at chromosome 22 (one red and green signal, (d)) or hemizygous deletion in NF2 (one red and two green signals, data not shown).
By univariate analysis, no statistically significant differences were identified between homozygous CDKN2A deletions or hemizygous NF2 loss and patient gender (P=0.802 and P=0.460, respectively), mean patient age (P=0.114 and P=0.750), asbestos exposure (P=0.371 and P=0.784), mean PCI (P=0.165 and P=0.151), incomplete cytoreduction (CC score of 2 to 3, P=1.000 and P=0.078), histologic subtype (P=1.000 and P=1.000), extent of invasion (P=0.815 and P=1.000), and lymph node metastasis (P=1.000 and P=1.000). Although there were no differences between homozygous CDKN2A deletions and mean age, patients with peritoneal mesothelioma harboring homozygous CDKN2A deletions were frequently ≥60 years of age (P=0.028). In addition, there was no association between homozygous CDKN2A deletions and hemizygous NF2 loss (P=0.434). Of note, the lack of association between homozygous CDKN2A deletions and sarcomatoid mesothelioma contrasts previous studies.36, 37 However, the small sample size of sarcomatoid mesotheliomas (n=2) within the study cohort may account for this discrepancy.
BAP1 Immunohistochemistry
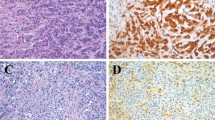
Loss of BAP1 nuclear protein expression was identified in 49 of 86 (57%) peritoneal mesotheliomas (Figure 2). Similar to CDKN2A and NF2, no statistically significant differences were identified between BAP1 status and patient gender (P=0.640), asbestos exposure (P=0.783), mean PCI (P=0.591), incomplete cytoreduction (CC score of 2 or 3, P=1.000), extent of invasion (P=0.828), and lymph node metastasis (P=0.315; Table 2). However, the absence of BAP1 correlated with increased mean patient age (57.0 years vs 48.3 years, P=0.006) and an epithelioid histologic subtype (98% vs 73%, P<0.001). Once again, the small sample size of non-epithelioid mesotheliomas within the study cohort should be noted. BAP1 loss was not associated with homozygous CDKN2A deletions (P=0.094), hemizygous NF2 loss (P=0.820), and losses in either gene (P=0.821) or both (P=1.000). Of note, 68 of 86 (79%) peritoneal mesotheliomas had a homozygous CDKN2A deletion, hemizygous NF2 loss and/or absent BAP1 nuclear expression.
BAP1 immunohistochemistry in peritoneal mesotheliomas. Preserved immunolabeling for BAP1 was defined as nuclear staining within tumor cells and stromal cells, which served as a positive internal control (a, H&E; b, BAP1). Cases with BAP1 loss showed absence of nuclear staining within neoplastic cells and preserved staining in the surrounding stroma (c, H&E; d, BAP1). H&E, hematoxylin and eosin.
Follow-up
Follow-up information was available for all patients and ranged from 2 to 153 months (mean, 33.5 months; median, 21.5 months). Tumor progression was identified in 60 (70%) patients. Eleven of 60 (18%) patients had sufficient pathologic material for repeat CDKN2A and NF2 fluorescence in situ hybridization, and BAP1 immunohistochemical testing. Comparative analyses demonstrated no differences between the primary mesothelioma and corresponding recurrence. Among all 86 patients, PFS and OS rates were 31% and 54% at 2-years with a median of 15 and 29 months, respectively.
Patients with homozygous CDKN2A deletions, hemizygous NF2 loss or both had decreased PFS and OS rates (Figure 3). Homozygous deletions in CDKN2A were associated with a 2-year PFS rate of 14% (vs 38%, P=0.013) with a median of 12 months and OS rate of 34% (vs 62%, P=0.026) with a median of 17 months. The 2-year PFS and OS rates for hemizygous loss of NF2 were 18% (vs 38%, P=0.010) with a median of 10 months and 33% (vs 66%, P=0.011) with a median of 21 months, respectively. Moreover, patients with mesotheliomas that harbored both homozygous CDKN2A deletions and hemizygous NF2 loss had an even shorter 2-year PFS rate of 9% with a median of 6 months (P=0.002) and OS rate of 18% with a median of 8 months (P=0.001). In contrast, no statistically significant differences in 2-year PFS and OS rates were observed based on the status of BAP1 (P=0.921 and P=0.780, respectively). As the majority of peritoneal mesotheliomas are epithelioid in histologic subtype and non-epithelioid peritoneal mesotheliomas are reported to be associated with a poor outcome, separate PFS and OS analyses were performed for epithelioid peritoneal mesotheliomas with respect to CDKN2A, NF2, and BAP1 status. No significant differences in PFS and OS were identified with inclusion of epithelioid peritoneal mesotheliomas alone in comparison with the entire study cohort.
Kaplan–Meier curves compare the cumulative probability for progression-free survival and overall survival among peritoneal mesotheliomas with homozygous CDKN2A deletions (a and b, respectively) and hemizygous NF2 loss (c and d, respectively). Patients with both homozygous CDKN2A deletion and hemizygous NF2 loss had a shorter progression-free survival (e), and worse overall survival (f) than patients with alterations in either gene alone. The P-values were calculated using a log-rank test.
In order to identify independent prognostic factors for patient PFS and OS, various clinicopathologic characteristics were evaluated using univariate and multivariate Cox proportional hazards regression models. By univariate analysis, a shorter PFS was associated with an age of ≥60 years (P=0.035), PCI (P=0.004), extension into the visceral parenchyma (P=0.038), and combined homozygous CDKN2A deletions and hemizygous NF2 loss (P=0.011). Worse OS correlated with age of ≥60 years (P=0.010), PCI (P=0.001), CC score of 2 to 3 (P=0.025), extension into the visceral parenchyma (P=0.024), and combined homozygous CDKN2A deletions and hemizygous NF2 loss (P=0.001; Table 3). Multivariate analysis was also used to determine the prognostic significance of CDKN2A and NF2 status for PFS and OS, and included age of ≥60 years, PCI, CC score of 2 to 3, extent of invasion, and combined homozygous CDKN2A deletions and hemizygous NF2 loss (Table 4). The combination of homozygous deletions in CDKN2A and hemizygous loss of NF2 was an independent prognostic factor for both PFS (P=0.019) and OS (P=0.001).
Discussion
Similar to their pleural counterparts, peritoneal mesotheliomas exhibit deletions or loss in CDKN2A (29%), NF2 (35%), and BAP1 (57%). However, the prevalence of homozygous CDKN2A deletions and hemizygous NF2 loss in peritoneal mesotheliomas is less than those reported for pleural mesotheliomas.16, 21, 22, 23, 26, 32 In addition, the most frequent abnormality in peritoneal mesotheliomas was the loss of BAP1 protein expression rather than homozygous CDKN2A deletions. An explanation for the disparities between peritoneal and pleural mesotheliomas remains elusive, but not surprising as both entities are clinically and pathologically distinct. The median patient age at diagnosis within our study cohort was 54 years, which is younger than the median patient age of 72 years for pleural mesotheliomas.38 Both peritoneal and pleural mesotheliomas occur predominantly in males, but a larger proportion of women develop peritoneal mesothelioma with a male-to-female ratio ranging between 2 and 3 to 1 (vs 4 and 5 to 1 for pleural mesotheliomas).1 Asbestos exposure is a risk factor for both peritoneal and pleural mesotheliomas. However, this association is weaker with peritoneal mesotheliomas.39 Although the histologic features for peritoneal mesotheliomas are generally identical to their pleural counterpart and divided into epithelioid, sarcomatoid, and biphasic subtypes, the vast majority of peritoneal mesotheliomas are epithelioid tumors.1 Last, differential RNA profiling and protein-expression analysis suggest a contrasting molecular pathogenesis between these two entities.40, 41
Despite the differences between peritoneal and pleural mesotheliomas, previous studies have demonstrated homozygous CDKN2A deletions in malignant mesothelioma are a poor prognostic indicator regardless of site.22, 25, 32 Consistent with these reports, we found that patients with peritoneal mesothelioma harboring a homozygous CDKN2A deletion had decreased PFS and OS. In addition, patients with a hemizygous NF2 loss also exhibited poor PFS and OS. Further, the significance of these findings was cumulative. Patients with both a homozygous CDKN2A deletion and a hemizygous NF2 loss had a worse clinical outcome than patients with alterations in either gene alone. The 2-year PFS and OS rates were 9 and 18%, respectively, with a median of 6 and 8 months, respectively. Similar parallels have been observed in experimental animal models. Both CDKN2A and NF2 encode for tumor-suppressor genes and when either gene is inactivated within a murine model, the mice rarely develop mesothelioma.28, 42 However, concomitant loss of both CDKN2A and NF2 results in a high incidence of mesothelioma with a relatively short latency.42 Taken together, these observations indicate alterations in both CDKN2A and NF2 define an aggressive subtype of mesothelioma.
With the introduction CRS and HIPEC, several studies have reported significant improvement in survival for patients with peritoneal mesothelioma. Nonetheless, the morbidity and mortality rates after CRS and HIPEC range from 15 to 31% and 0 to 7%, respectively.10, 13, 14, 15 Consequently, various staging systems have been proposed to identify appropriate surgical candidates, stratify treatment regimens and more accurately predict prognosis.43, 44 Although the specific clinical and pathologic parameters differ for each system, they primarily evaluate three aspects of the patient’s disease: (i) the presence of extra-abdominal metastases; (ii) extent of tumor burden (e.g., based on imaging studies or PCI); and (iii) individual prognostic variables including patient age, histologic subtype, nuclear grade, mitotic count, depth of invasion (>0.5 mm), lymph node metastasis, and/or CC score. Only a few studies have examined the pathologic prognostic factors for patients treated with CRS and HIPEC. Many of these pathologic findings can be challenging to interpret, subjective in grading or rarely identifiable to be of clinical significance. In comparison, homozygous deletions in CDKN2A and hemizygous loss of NF2, as assessed by fluorescence in situ hybridization, represent objective and reproducible prognostic biomarkers for peritoneal mesotheliomas. Moreover, by multivariate analysis, the presence of both a homozygous CDKN2A deletion and a hemizygous NF2 loss was an independent prognostic factor for shorter PFS and poor OS. In fact, median OS for patients with peritoneal mesothelioma harboring homozygous deletions in CDKN2A and hemizygous loss of NF2 was similar to the reported survival before the introduction of CRS and HIPEC therapy. Thus, considering the negative prognostic implications, patients with peritoneal mesothelioma harboring these alterations may not benefit from aggressive CRS and HIPEC, and warrants additional studies.
Loss of BAP1 nuclear expression in peritoneal mesotheliomas did not correlate with changes in PFS or OS. In comparison, the significance of BAP1 alterations in pleural mesotheliomas has become a topic of contention. Initial studies reported BAP1 mutations occurred in 20% of pleural mesotheliomas and were not associated with differences in OS.17, 45 Recently, Nasu et al18 found that combining multiple molecular techniques identified BAP1 alterations in 63.6% of pleural mesotheliomas. Further, the authors concluded immunohistochemistry for BAP1 nuclear expression was the most reliable method of assessing BAP1 status. Within two large, independent cohorts of pleural mesotheliomas, Farzin et al30 and McGregor et al46 identified loss of BAP1 nuclear expression in 46.3% and 48% of pleural mesotheliomas, respectively. In both studies, BAP1 loss predicted improved OS. However, according to McGregor et al,46 this association was not prognostically significant when only cases of the epithelioid histologic subtype were analyzed.46 As the majority of peritoneal mesotheliomas are histologically epithelioid, this may account for the absence of a clear survival benefit for BAP1 loss.
Nonetheless, the present study is not without limitations. It is retrospective by design and not all patients were treated the same. Although every patient within our cohort underwent CRS and HIPEC, 20% of patients received preoperative systemic chemotherapy. Historically, treatment modalities for peritoneal mesothelioma included systemic chemotherapy and palliative surgery, but all patients eventually died from the disease with a median survival of <1 year.47 Thus, traditional systemic chemotherapeutic options for peritoneal mesothelioma are generally considered to be ineffective. In addition, the sample size of non-epithelioid peritoneal mesotheliomas within this study was quite small. Previous studies have demonstrated a strong association between sarcomatoid mesotheliomas and homozygous CDKN2A deletions.36, 37 Although a similar association was not identified herein, the presence of only two sarcomatoid mesotheliomas within our cohort may account for this discrepancy. However, as previously mentioned, the vast majority of peritoneal mesotheliomas are epithelioid in histologic subtype.
With respect to biomarker detection, the growing knowledge of multiple molecular alterations that contribute to tumor pathophysiology has begun to shift toward clinical testing to focus on the genetic techniques that can interrogate a larger proportion of the cancer genome in an unbiased fashion. Several high-throughput molecular tests, such as array-based comparative genome hybridization, single-nucleotide pleomorphism arrays, and next-generation sequencing, have recently been incorporated into routine clinical practice and, in some laboratories, replaced the classical assays, such as fluorescence in situ hybridization. However, the simplicity and reliability of fluorescence in situ hybridization to detect specific genomic alterations makes it an invaluable diagnostic tool. Fluorescence in situ hybridization does not require tissue processing and/or amplification of tumor DNA and/or RNA. It can be directly performed on fresh or FFPE tissue for rapid evaluation of tumor interphase nuclei, and is ideal for small biopsies that are often encountered with peritoneal lesions. Hence, until the emergence of further advancements in molecular techniques, fluorescence in situ hybridization is expected to continue to have a vital role in the assessment of peritoneal mesotheliomas.
In summary, we report the assessment of CDKN2A, NF2, and BAP1 status in a large cohort of peritoneal mesotheliomas. The combination of a homozygous CDKN2A deletion and a hemizygous NF2 loss in peritoneal mesotheliomas was an independent prognostic factor for both shorter PFS and poor OS. In contrast, loss of BAP1 protein expression was not associated with changes in clinical outcome. Although further studies are required, CDKN2A and NF2 fluorescence in situ hybridization analysis may guide treatment decisions for patients with malignant peritoneal mesothelioma.
References
Hesdorffer ME, Chabot J, DeRosa C et al. Peritoneal mesothelioma. Curr Treat Options Oncol 2008;9:180–190.
Price B, Ware A . Mesothelioma trends in the United States: an update based on Surveillance, Epidemiology, and End Results Program data for 1973 through 2003. Am J Epidemiol 2004;159:107–112.
Bridda A, Padoan I, Mencarelli R et al. Peritoneal mesothelioma: a review. MedGenMed 2007;9:32.
Acherman YI, Welch LS, Bromley CM et al. Clinical presentation of peritoneal mesothelioma. Tumori 2003;89:269–273.
Antman KH . Current concepts: malignant mesothelioma. N Engl J Med 1980;303:200–202.
Antman KH, Osteen RT, Klegar KL et al. Early peritoneal mesothelioma: a treatable malignancy. Lancet 1985;2:977–981.
Sugarbaker PH . Management of peritoneal-surface malignancy: the surgeon's role. Langenbecks Arch Surg 1999;384:576–587.
Yan TD, Welch L, Black D et al. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol 2007;18:827–834.
Alexander HR Jr., Bartlett DL, Pingpank JF et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013;153:779–786.
Feldman AL, Libutti SK, Pingpank JF et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560–4567.
Magge D, Zenati MS, Austin F et al. Malignant peritoneal mesothelioma: prognostic factors and oncologic outcome analysis. Ann Surg Oncol 2014;21:1159–1165.
Loggie BW, Fleming RA, McQuellon RP et al. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg 2001;67:999–1003.
Deraco M, Nonaka D, Baratti D et al. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol 2006;13:229–237.
Yan TD, Deraco M, Baratti D et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237–6242.
Sugarbaker PH, Welch LS, Mohamed F et al. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am 2003;12:605–621.
Guo G, Chmielecki J, Goparaju C et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res 2015;75:264–269.
Bott M, Brevet M, Taylor BS et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668–672.
Nasu M, Emi M, Pastorino S et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565–576.
Cheng JQ, Jhanwar SC, Klein WM et al. p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res 1994;54:5547–5551.
Bianchi AB, Mitsunaga SI, Cheng JQ et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci USA 1995;92:10854–10858.
Illei PB, Rusch VW, Zakowski MF et al. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res 2003;9:2108–2113.
Dacic S, Kothmaier H, Land S et al. Prognostic significance of p16/cdkn2a loss in pleural malignant mesotheliomas. Virchows Arch 2008;453:627–635.
Chiosea S, Krasinskas A, Cagle PT et al. Diagnostic importance of 9p21 homozygous deletion in malignant mesotheliomas. Mod Pathol 2008;21:742–747.
Kobayashi N, Toyooka S, Yanai H et al. Frequent p16 inactivation by homozygous deletion or methylation is associated with a poor prognosis in Japanese patients with pleural mesothelioma. Lung Cancer 2008;62:120–125.
Lopez-Rios F, Chuai S, Flores R et al. Global gene expression profiling of pleural mesotheliomas: overexpression of aurora kinases and P16/CDKN2A deletion as prognostic factors and critical evaluation of microarray-based prognostic prediction. Cancer Res 2006;66:2970–2979.
Thurneysen C, Opitz I, Kurtz S et al. Functional inactivation of NF2/merlin in human mesothelioma. Lung Cancer 2009;64:140–147.
Xiao GH, Gallagher R, Shetler J et al. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol 2005;25:2384–2394.
Fleury-Feith J, Lecomte C, Renier A et al. Hemizygosity of Nf2 is associated with increased susceptibility to asbestos-induced peritoneal tumours. Oncogene 2003;22:3799–3805.
Sheffield BS, Hwang HC, Lee AF et al. BAP1 Immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol 2015;39:977–982.
Farzin M, Toon CW, Clarkson A et al. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology 2015;47:302–307.
Baumann F, Flores E, Napolitano A et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76–81.
Krasinskas AM, Bartlett DL, Cieply K et al. CDKN2A and MTAP deletions in peritoneal mesotheliomas are correlated with loss of p16 protein expression and poor survival. Mod Pathol 2010;23:531–538.
Jacquet P, Sugarbaker PH . Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359–374.
Mohamed F, Cecil T, Moran B et al. A new standard of care for the management of peritoneal surface malignancy. Curr Oncol 2011;18:e84–e96.
Chung CT, Santos Gda C, Hwang DM et al. FISH assay development for the detection of p16/CDKN2A deletion in malignant pleural mesothelioma. J Clin Pathol 2010;63:630–634.
Tochigi N, Attanoos R, Chirieac LR et al. p16 Deletion in sarcomatoid tumors of the lung and pleura. Arch Pathol Lab Med 2013;137:632–636.
Wu D, Hiroshima K, Matsumoto S et al. Diagnostic usefulness of p16/CDKN2A FISH in distinguishing between sarcomatoid mesothelioma and fibrous pleuritis. Am J Clin Pathol 2013;139:39–46.
Tsao AS, Wistuba I, Roth JA et al. Malignant pleural mesothelioma. J Clin Oncol 2009;27:2081–2090.
Browne K, Smither WJ . Asbestos-related mesothelioma: factors discriminating between pleural and peritoneal sites. Br J Ind Med 1983;40:145–152.
Borczuk AC, Cappellini GC, Kim HK et al. Molecular profiling of malignant peritoneal mesothelioma identifies the ubiquitin-proteasome pathway as a therapeutic target in poor prognosis tumors. Oncogene 2007;26:610–617.
Trupiano JK, Geisinger KR, Willingham MC et al. Diffuse malignant mesothelioma of the peritoneum and pleura, analysis of markers. Mod Pathol 2004;17:476–481.
Jongsma J, van Montfort E, Vooijs M et al. A conditional mouse model for malignant mesothelioma. Cancer Cell 2008;13:261–271.
Deraco M, Bartlett D, Kusamura S et al. Consensus statement on peritoneal mesothelioma. J Surg Oncol 2008;98:268–272.
Yan TD, Deraco M, Elias D et al. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database*. Cancer 2011;117:1855–1863.
Zauderer MG, Bott M, McMillan R et al. Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J Thorac Oncol 2013;8:1430–1433.
McGregor S, Dunning R, Hadi D et al. BAP1 loss portends improved prognosis in malignant pleural mesothelioma due to frequent association with epithelioid morphology. Mod Pathol 2015;28:484A.
Munkholm-Larsen S, Cao CQ, Yan TD . Malignant peritoneal mesothelioma. World J Gastrointest Surg 2009;1:38–48.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Singhi, A., Krasinskas, A., Choudry, H. et al. The prognostic significance of BAP1, NF2, and CDKN2A in malignant peritoneal mesothelioma. Mod Pathol 29, 14–24 (2016). https://doi.org/10.1038/modpathol.2015.121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2015.121
This article is cited by
-
Well differentiated papillary mesothelial tumor: a new name and new problems
Modern Pathology (2022)
-
Solid papillary mesothelial tumor
Modern Pathology (2022)
-
Malignant peritoneal mesothelioma: prognostic significance of clinical and pathologic parameters and validation of a nuclear-grading system in a multi-institutional series of 225 cases
Modern Pathology (2021)
-
Female adnexal tumors of probable Wolffian origin: morphological, immunohistochemical, and molecular analysis of 15 cases
Modern Pathology (2020)
-
Cyclin D1 immunohistochemical staining to separate benign from malignant mesothelial proliferations
Modern Pathology (2020)