Abstract
Traditional serrated adenoma is one type of colorectal serrated neoplasm and a precursor of colorectal cancer. We evaluated the pathologic and molecular features of 60 traditional serrated adenomas with cytologic dysplasia and/or invasive carcinoma. On the basis of morphological features, 16 cases (27%) were categorized as traditional serrated adenoma with serrated dysplasia and 25 cases (42%) as traditional serrated adenoma with conventional adenomatous dysplasia. In addition, 19 cases (31%) showed an overall tubulovillous adenomatous structure but with focal serrated feature. Traditional serrated adenoma with serrated dysplasia had a significantly higher frequency of BRAF mutation than traditional serrated adenoma with conventional adenomatous dysplasia and tubulovillous adenoma with serrated feature (P=0.006), whereas traditional serrated adenoma with conventional adenomatous dysplasia and tubulovillous adenoma with serrated feature had higher frequencies of KRAS mutation than traditional serrated adenoma with serrated dysplasia (P<0.0001). Only traditional serrated adenoma with serrated dysplasia showed sessile serrated adenoma-like lesions at the periphery (n=3) and developed invasive carcinomas when the lesions were <15 mm in size. Abnormal nuclear accumulation of β-catenin was detected in traditional serrated adenoma with conventional adenomatous dysplasia and tubulovillous adenoma with serrated feature but not in traditional serrated adenoma with serrated dysplasia. The frequency of the positive CpG island methylator phenotype was similar among the three dysplastic subtypes, and immunostaining of four mismatch repair proteins in the nucleus was retained in all traditional serrated adenomas and associated invasive malignancies. Traditional serrated adenoma-associated adenocarcinomas (n=28) displayed distinctive morphological features: oval cell nuclei, serrated glands, infiltrating borders, rare occurrences of necrosis and mucinous differentiation. Overexpression of p53 was detected only in high-grade dysplasia and invasive adenocarcinoma. Our findings indicate that traditional serrated adenoma is a heterogeneous neoplasm with two pathways of neoplastic progression, which are distinct from the sessile serrated pathway of colorectal carcinogenesis.
Similar content being viewed by others
Main
Traditional serrated adenoma is a rare type of serrated polyp that accounts for <1% of all colorectal polyps.1 Traditional serrated adenoma constitutes one major category of serrated neoplasms and was initially termed ‘serrated adenoma’ by Longacre et al in 1990.2 However, the terminology was confusing because it also included sessile serrated adenoma in previous literatures.1, 3, 4, 5
The criteria for diagnosing traditional serrated adenoma were not standardized until the comprehensive work of Torlakovic et al in 2008.6 Traditional serrated adenoma is characterized by its predominance in the left-sided colon, a villiform and protuberant growth pattern, serrated contours, and columnar lining cells with eosinophilic cytoplasm and oval to slender nuclei.6 The feature more useful for differentiating traditional serrated adenoma from other serrated polyps is the presence of small ectopic crypts, which are not anchored to the muscularis mucosae.1, 2, 6 Although traditional serrated adenoma shares some pathologic features with sessile serrated adenoma,7, 8 and it is not uncommon to see a sessile lesion resembling sessile serrated adenoma in the periphery of traditional serrated adenoma,7, 9 traditional serrated adenoma differs from sessile serrated adenoma in many pathologic and molecular aspects. Traditional serrated adenoma displays a protuberant growth pattern and is detected more frequently in the left-sided colon, and 29–46% of traditional serrated adenomas harbor KRAS mutation, which rarely occurs in sessile serrated adenoma.7, 10, 11 Sessile serrated adenoma is a recognized precursor of colorectal cancer with high levels of microsatellite instability,1, 9 whereas traditional serrated adenoma is more likely to evolve into a colorectal cancer that is microsatellite-stable or has low levels of microsatellite instability.1, 9, 11 Furthermore, the Wnt signaling pathway was shown to be activated in a subset of traditional serrated adenoma.12 Thus, traditional serrated adenoma is a heterogeneous entity with partial molecular features of the sessile serrated pathway and the conventional adenoma–carcinoma sequence.13 However, the genetic changes of traditional serrated adenoma carcinogenesis remain to be established.
Sporadic colorectal cancer with high levels of microsatellite instability are a prototype of the sessile serrated pathway to colorectal cancer and are considered to arise from sessile serrated adenoma.1, 9 Microsatellite-unstable status is caused by epigenetic inactivation of the MLH1 via hypermethylation of the promoter CpG island. Recent data have suggested the existence of a second serrated pathway to colon carcinoma: the cancers in this subset of colon cancer are often microsatellite-stable or have low levels of microsatellite instability with either BRAF or KRAS mutation.5, 9, 14, 15, 16 Some of the cancers in the second subset of colon cancers meet the morphological criteria of serrated adenocarcinoma, and serrated adenocarcinoma has been reported to develop from traditional serrated adenoma.14 Thus, traditional serrated adenoma may represent a distinct serrated precursor in colorectal carcinogenesis with a unique molecular pathogenesis.
Two major patterns of cytologic dysplasia were reported in traditional serrated adenoma: serrated dysplasia and conventional adenomatous dysplasia.1 However, the biological significance of these two morphological patterns is still unknown. Although some pathologists diagnose tumors with combined features as mixed hyperplastic/adenomatous polyps or mixed polyps with a description of the constituent components,17, 18 mixed polyps have also been reported to share similar molecular changes and implicate morphological progression to malignancy.13, 19 Jass et al13 reported that traditional serrated adenoma with dysplastic epithelium resembling conventional adenoma displayed a high frequency of KRAS mutation, whereas Kim et al7 linked this traditional serrated adenoma to a lower frequency of BRAF mutation. By contrast, no genetic changes were correlated to the dysplastic features of traditional serrated adenoma by Fu et al.10 This discrepancy may be due to the use of different diagnostic criteria for classifying the two morphological patterns of cytologic dysplasia. Furthermore, some lesions have an overall tubulovillous adenomatous structure but a focally discernible traditional serrated adenoma morphology. This lesion is ambiguous in the current classification system because biological significance and molecular features are unknown.
In this study, we investigated a series of traditional serrated adenomas with cytologic dysplasia and/or adenocarcinomatous transformation focusing on the molecular genetics and the pathologic features of the traditional serrated adenomas. Our goal is to elucidate the heterogeneity in the genetic–epigenetic events in, and the biologic uniqueness of, the neoplastic pathways leading from traditional serrated adenoma to colon cancer.
Materials and methods
Case Selection
We surveyed 4061 colorectal carcinomas resected from January 2005 to June 2013 at the National Taiwan University Hospital by reviewing the pathology reports. A total of 796 cases harboring residual precursor lesions were retrieved for slide review (by two pathologists, JH Tsai and Y-M Jeng), and 28 colorectal adenocarcinomas were identified as arising in conjunction with traditional serrated adenoma. We also included 32 more traditional serrated adenomas with cytologic dysplasia but without invasive cancer in this study. We followed the histological criteria proposed by Torlakovic et al6 for diagnosing traditional serrated adenoma. Traditional serrated adenoma is defined as a precursor lesion with serrated contours and is covered by columnar epithelial cells with dense eosinophilic cytoplasm.1, 6 The nuclei are slender and basal located. Two types of serration occur in traditional serrated adenoma: narrow indentations of the luminal border of the lining cells and concave depressions of the surface epithelium because of the formation of ectopic crypts.9 Cases with a complex villiform architecture with bulbous projection and a sessile growth pattern in part of the tumor are included. Low-grade (mild and moderate) and high-grade (severe) dysplasia were classified based on both cytologic changes and architectural complexity.1, 20 Two morphological patterns of cytologic dysplasia were recognized based on the criteria proposed by Goldstein: serrated dysplasia and conventional adenomatous dysplasia.21 Traditional serrated adenomas with both serrated-type and conventional adenomatous dysplasia were grouped into traditional serrated adenoma with conventional adenomatous dysplasia. A third group of traditional serrated adenomas showing conventional adenomatous dysplasia in most parts of the tumor but with focal traditional serrated adenoma components was designated as tubullovillous adenoma with serrated feature.
The following pathologic features were recorded: maximal diameter of precursor lesions (measured on glass slides), characteristic features of colon cancer with high levels of microsatellite instability (pushing tumor border, peri-tumor Crohn-like reaction and tumor-infiltrating lymphocytes), luminal necrosis, mucinous differentiation in the invasive carcinoma9, 22 and serration in the adenocarcinoma component.1, 9, 23 Tumor location was divided as right side (cecum, ascending and transverse colon) and left side (descending, sigmoid colon and rectum).
This study was conducted according to the regulations of the Research Ethics Committee of National Taiwan University Hospital, and the specimens were rendered anonymous and evaluated in a blinded manner.
Immunohistochemistry
Tissue sections (5-μm thick) were cut from paraffin blocks and the slides were dewaxed and rehydrated. Antigens were retrieved by incubating the slides in Epitope Retrieval 2 solution (pH 9.0, Leica Biosystems, Newcastle, UK) at 100 °C for 10 min. Sections were stained on a Leica Microsystems Bondmax autostainer, according to the manufacturer’s protocol. Primary antibodies against the following proteins were used: p53 (clone D07, 1:50; Dako Cytomation, Carpinteria, CA, USA), β-catenin (1:200; Dako Cytomation), MLH1 (clone ES05, pre-diluted; Leica Biosystems), MSH2 (clone 25D12, pre-diluted; Leica Biosystems), PMS2 (clone A16-4, 1:100; BD Pharmingen, CA, USA) and MSH6 (clone EPR3945, 1:100; Abcam, Cambridge, MA, USA). Labeling for β-catenin was defined as abnormal if membrane staining was lost or nuclear β-catenin expression was detected. Strong nuclear expression for p53 in 50% or more of the abnormal epithelium was considered a positive result. For mismatch repair proteins, the absence of nuclear expression in >50% of the tumor cells was defined as a negative result.
Molecular Analysis
The traditional serrated adenoma lesions including the dysplastic and non-dysplastic components from 10-μm paraffin sections were dissected using sterilized razors under a microscope. Genomic DNA was extracted using a QIAamp DNA FFPE Tissue Kit (Qiagen, Santa Clarita, CA, USA) according to the manufacturer’s protocol. The samples were subjected to polymerase chain reaction, using pairs of primers encompassing exon 15 of BRAF: (5′-TCATAATGCTTGCTCTGATAGGA-3′) and 5′- GGCCAAAAATTTAATCAGTGGA-3′; and exon 2 of KRAS: (5′-GAATGGTCCTGCACCAGTAA-3′; and 5′-GTGTGACATGTTCTAATATAGTCA-3′). The amplified DNA fragments were purified and sequenced directly using an automated ABI 3730 sequencer (Applied BioSystems, Foster City, CA, USA).
CpG Island Methylator Phenotype
Five marker loci (p16, MINT1, MINT2, MINT31 and MLH1) were selected to evaluate their methylation status using the Methylight assay. Briefly, genomic DNA was treated with sodium bisulfite following the recommendations of the EZ DNA Methylation Kit protocol (Zymo Research, Orange, CA, USA). The normalization control reaction was based on methylation-independent measurement of Alu repeats. The methylation levels were measured for the five CpG island methylator phenotype loci in the samples and a constant reference sample were subjected to quantitative DNA methylation analysis. The percentage of methylated reference was calculated using the equation provided previously,24 and loci were considered to be methylated if the percentage of methylated reference was >10. CpG island methylator phenotype was designated as positive if 3 or more of methylated loci were identified and negative in the cases with <3 methylated loci.
Statistical Analysis
Data were analyzed using SPSS 16.0 software (Chicago, IL, USA). Categorical variables were compared using the Pearson χ2 method and continuous variables were analyzed using Student’s t-test.
Results
Morphological Subtypes of Traditional Serrated Adenoma with Neoplastic Progression
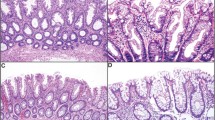
The 60 traditional serrated adenomas with cytologic dysplasia examined in this study were categorized into three morphological subtypes: traditional serrated adenoma with serrated dysplasia (n=16), traditional serrated adenoma with conventional adenomatous dysplasia (n=25) and tubullovillous adenoma with serrated feature (n=19). Components of traditional serrated adenoma (Figures 1a and b) were detected in all cases, but their percentage varied. The epithelium showing serrated dysplasia was composed of columnar cells with serrated contours, high nucleocytoplasmic ratio, open chromatin, irregularly thickened nuclear membrane and dense eosinophilic cytoplasm (Figure 1c). High-grade serrated dysplasias harbored complex structural changes such as micropapillary protrusions, crowded ectopic crypts and cribriform bridging of glands, and occasionally displayed prominent nucleoli (Figure 1d). The dysplastic components in traditional serrated adenoma with conventional adenomatous dysplasia were characterized by elongated and enlarged nuclei with nuclear pseudostratification, hyperchromasia and tubulovillous growth, reminiscent of conventional tubulovillous adenoma (Figure 1e). Seven out of the 25 (28%) traditional serrated adenomas with conventional adenomatous dysplasia showed combined serrated and conventional adenomatous dysplasia. Cases of tubullovillous adenoma with serrated feature displayed an overall tubulovillous adenomatous dysplasia with recognizable traditional serrated adenoma components (Figure 1f). The demographic and pathological data of traditional serrated adenomas with cytologic dysplasia are shown in Table 1. No statistically significant differences were detected in age, sex or location among the three subtypes. All three subtypes of traditional serrated adenomas with cytologic dysplasia occurred preferentially in the left-sided colon. Thirty-five cases (58%) exhibited high-grade dysplasia and 28 cases showed invasive adenocarcinoma (47%). The mean sizes of the precursor polyps with and without the invasive carcinoma part were 21 and 14 mm, respectively (P=0.002). Interestingly, in cases with invasive carcinoma, the polyps in traditional serrated adenoma with serrated dysplasia were significantly smaller than polyps in the other two subtypes (P=0.023). The majority of traditional serrated adenomas with cytologic dysplasia (80%) showed a low pathologic tumor stage (pT0–T2), most likely because of the preserved precursor polyps being included in this study. Operative methods for the 60 cases were the following: right hemicolectomy, 15; low anterior resection, 9; left hemicolectomy, 1; endoscopic mucosal resection, 16; and polypectomy, 19.
Traditional serrated adenoma with cytologic dysplasia. (a) A traditional serrated adenoma with villous growth and bulbous villous tips. The lining cells showed slender columnar cells with eosinophilic cytoplasm. (b) Prominent surface serration with many abortive ectopic crypts (arrows). (c) A traditional serrated adenoma with high-grade serrated dysplasia. Note the complex infoldings of ectopic crypts and occasional micropapillary structures. (d) At higher power, the cells show oval nuclei, vesicular chromatin, thickened nuclear membrane and occasional prominent nucleoli. (e) A traditional serrated adenoma (left part) showed transition to conventional adenomatous dysplasia at right part of the figure (arrows). (f) Traditional serrated adenoma with an overall conventional tubulovillous adenomatous structure. Focal discernible traditional serrated adenoma component was seen at left part of the figure (arrows).
Morphological Features of Invasive Carcinoma Arising from Traditional Serrated Adenoma
In the 28 invasive adenocarcinomas arising from traditional serrated adenoma, several characteristic pathologic features were observed (Table 1). Tumor cells often showed oval or low columnar nuclei with an eosinophilic cytoplasm and at least a focally serrated morphology. Extracellular mucinous components were found in 46% of traditional serrated adenomas with invasive carcinoma (Figure 2a). More than 90% of the invasive adenocarcinomas harbored infiltrating borders with small tumor nests in the invading edge (Figure 2b). Tumor necrosis in >10% of the lesion was detected in only two cases (3%) and the prominent ‘dirty’ necrosis commonly seen in conventional colorectal carcinoma was not identified in any of the cases. Eleven out of the 28 cases (39%) were diagnosed as serrated adenocarcinoma by the morphological criteria of epithelial serration, abundant eosinophilic cytoplasm and <10% necrosis9, 23 (Figure 2c). The tumor cells of the serrated adenocarcinomas typically had vesicular nuclei with a condensed nuclear membrane and distinct single nucleoli (Figure 2d). Three of the 11 cases were recognized as the mucinous type of serrated adenocarcinoma. The morphological features of colorectal cancer with high levels of microsatellite instability were detected in only a small subset of the traditional serrated adenoma-associated invasive carcinomas: peri-tumoral Crohn-like reaction (n=7), tumor-infiltrating lymphocytes (n=9) and pushing tumor border (n=3). None of the cases showed all three morphological features.
Traditional serrated adenoma-associated invasive adenocarcinoma had common characteristic pathologic features: Extracellular mucinous differentiation (a) and infiltrating tumor border at invasive fronds (b). (c, d) A serrated adenocarcinoma arising from a traditional serrated adenoma showed serrated contour, abundant eosinophilic cytoplasm and vesicular nuclei with single prominent nucleoli.
Immunohistochemical Analysis of Traditional Serrated Adenoma with Cytologic Dysplasia
Strong and abnormal nuclear immunostaining of p53 was detected in 19 cases (31%), and the staining was limited to areas of high-grade dysplasia and invasive growth (Figures 3a and b). The aberrant nuclear expression of p53 did not differ among the three subtypes (Table 2). Loss of β-catenin staining at the cell membrane or the abnormal nuclear expression of β-catenin was detected in traditional serrated adenoma with conventional adenomatous dysplasia (n=2) and tubullovillous adenoma with serrated feature (n=2) but not in traditional serrated adenoma with serrated dysplasia (Figures 3c and d). Mismatch repair proteins (MLH1, MSH2, PMS2 and MSH6) were diffusely expressed in nuclei in all cases including the invasive adenocarcinoma parts (Figure 3e).
Immunohistochemistry of traditional serrated adenoma with cytologic dysplasia. (a, b) Overexpression of p53 was present in the invasive adenocarcinoma part. (c) A traditional serrated adenoma showing conventional adenomatous dysplasia at lower part of the figure (arrows) (d) Abnormal β-catenin nuclear accumulation was noted at the adenomatous dysplasia. (e) Diffuse nuclear expression for the mismatch repair proteins (MLH1, PMS2, MSH2 and MSH6) was detected in all cases of traditional serrated adenomas with cytologic dysplasia and associated invasive malignancies.
Molecular Features of Traditional Serrated Adenoma with Cytologic Dysplasia
BRAF and KRAS mutations were detected in a mutually exclusive manner in 35% and 52% of traditional serrated adenomas with cytologic dysplasia, respectively (Table 2). The BRAF mutation frequency was highest in traditional serrated adenoma with serrated dysplasia (63%), lower in traditional serrated adenoma with conventional adenomatous dysplasia (36%) and lowest in tubullovillous adenoma with serrated feature (11%; P=0.006). By contrast, the KRAS mutation frequency was highest in tubullovillous adenoma with serrated feature (79%) and lowest in traditional serrated adenoma with serrated dysplasia (13%; P<0.0001). Of the seven cases of traditional serrated adenomas with combined serrated dysplasia and conventional adenomatous dysplasia, one (14%) was wild type in both genes, three (43%) harbored the KRAS mutations and three (43%) had the BRAF mutations. No association was found between the predominant dysplasia pattern and KRAS or BRAF mutation status in these seven cases. The KRAS mutation rate of traditional serrated adenomas showing combined dysplastic features was more similar to traditional serrated adenoma with conventional adenomatous dysplasia group than to traditional serrated adenoma with serrated dysplasia group. Sessile serrated adenoma-like morphology was detected at the edges in three cases of traditional serrated adenoma with serrated dysplasia. Interestingly, all the three cases harbored the BRAF mutation. Positive CpG island methylator phenotype status was identified in 57% of the traditional serrated adenomas with cytologic dysplasia and its prevalence did not differ among the three subtypes (P=0.544).
Characteristics of Molecular Pathology of Traditional Serrated Adenoma with Invasion
We compared immunohistochemical and molecular features of traditional serrated adenoma with and without invasive carcinoma (Table 3). Presence of p53 overexpression was significantly associated with invasive carcinoma (P=0.004). The four cases with abnormal nuclear expression of β-catenin harbored components of invasive malignancies. In contrast, mutations of KRAS, BRAF and CpG island methylator phenotype status were not correlated with the presence of invasive carcinoma.
Discussion
In this study, we collected a large series of traditional serrated adenomas with cytologic dysplasia and correlated the morphology of the dysplastic lesions with their immunohistochemical and molecular features. Although malignant transformation has been observed in traditional serrated adenomas,5, 7, 14, 23 the molecular and morphological details have not been described and the distinction from sessile serrated adenoma has not been addressed. Activation of the mitogen-activated protein kinase (MAPK) pathway, by either KRAS or BRAF mutation, was reported in 75 to 84% of traditional serrated adenomas7, 10 and was detected in approximately 87% of the traditional serrated adenomas with cytologic dysplasia in this study. Activation of MAPK signaling represents one of the most frequent molecular derangements in early tumorigenesis of traditional serrated adenoma.9 Overexpression of p53 is commonly observed in traditional serrated adenoma with cytologic dysplasia, especially in areas of high-grade dysplasia and invasive carcinoma. Abnormal nuclear accumulation of p53 is associated with missense mutation in p53,5, 25, 26 a tumor-suppressor gene that is typically disrupted late in the multistep carcinogenesis in the conventional adenoma–carcinoma sequence.1 Inactivation of p53 in traditional serrated adenoma may serve as a tool to break ras or raf activation-induced cell senescence and cell cycle arrest.27, 28 The correlation of immunoreactivity of p53 with high-grade dysplasia and invasive carcinoma indicates that p53 expression is a late event in the stepwise oncogenic process that occurs after the activation of the MAPK pathway in traditional serrated adenoma.
The four mismatch repair proteins (MLH1, MSH2, PMS2 and MSH6) were retained in all cases of traditional serrated adenomas with cytologic dysplasia, including those with invasive malignancy. Loss of immunostaining for mismatch repair proteins is a reliable test for microsatellite instability in colon cancer.22 Thus, our results suggest that most, if not all, colon cancers arising from traditional serrated adenoma were microsatellite-stable or had low levels of microsatellite instability, which agrees with the observations that traditional serrated adenoma is a precursor polyp of colon cancers with stable/low levels of microstallite instability and that MLH1 is rarely methylated in traditional serrated adenoma.1 However, this is in contrast with sessile serrated adenoma, which frequently shows loss of immunostaining for mismatch repair proteins in the invasive part of the tumor.29 Therefore, the traditional serrated adenoma pathway is distinct from the sessile serrated pathway and serve as an alternative route in serrated carcinogenesis. Consistent with these findings, we found that traditional serrated adenoma-associated carcinomas showed no combined characteristics of microsatellite-unstable colorectal carcinomas, such as a pushing border, a peri-tumoral Crohn-like inflammatory infiltrate and tumor-infiltrating lymphocytes,9, 22 but instead displayed the unique features of serrated configuration, oval nuclei, infiltrating tumor border, rare occurrences of necrosis and frequent mucinous differentiation. Thus, many of the invasive carcinomas in our series were diagnosed as serrated adenocarcinomas. Most serrated adenocarcinomas examined previously were microsatellite-stable or had low levels of microsatellite instability with KRAS or BRAF mutation,14, 23 which are molecular features similar to those of the traditional serrated adenomas with cytologic dysplasia studied here. Traditional serrated adenoma is therefore likely to be a precursor of serrated adenocarcinoma.
A key finding of this study was that the two morphological patterns of the cytologic dysplasia of traditional serrated adenoma had distinct features: traditional serrated adenoma with serrated dysplasia was highly associated with the BRAF mutation and a smaller polyp size, whereas traditional serrated adenoma with conventional adenomatous dysplasia and tubullovillous adenoma with serrated feature harbored the KRAS mutation and were larger. Jass et al13 also observed that traditional serrated adenomas and mixed polyps with serrated features of dysplasia (designated as ’group A‘ in their study) were associated with the BRAF mutation, whereas conventional adenomatous dysplasias (‘group B’) were highly associated with KRAS mutation. Kim et al7 observed a considerably lower rate of BRAF mutation in traditional serrated adenomas with conventional adenomatous dysplasia. The KRAS mutation was closely related to villous growth in colon adenomas,30, 31 and thus the mutation may confer a potential of protuberant growth, larger size and adenomatous dysplasia in traditional serrated adenoma. Interestingly, aberrant nuclear expression of β-catenin was detected in traditional serrated adenoma with conventional adenomatous dysplasia/tubullovillous adenoma with serrated feature but not in traditional serrated adenoma with serrated dysplasia. These findings suggest that traditional serrated adenoma with KRAS mutation developed conventional adenomatous dysplasia followed by the activation of the Wnt pathway and p53 derangement, similar to the conventional adenoma–carcinoma sequence.
Conversely, traditional serrated adenomas with BRAF mutation more commonly acquired serrated dysplasia than other traditional serrated adenomas, and our collection of traditional serrated adenoma with serrated dysplasia developed invasive carcinomas in tumors that were significantly smaller than in the other subtypes. The occurrence of invasive carcinoma in smaller lesion is similar to the rapid malignant change observed in sessile serrated adenoma with cytologic dysplasia.1, 32 We did not detect a higher frequency of traditional serrated adenoma with serrated dysplasia in traditional serrated adenoma-associated carcinomas. A possible explanation is that traditional serrated adenomas with serrated dysplasia developed invasive carcinomas when the polyps were small and were easily overgrown by the cancer, and thus were poorly detected. This possibility is supported by the observation that colon cancers with KRAS mutation are more likely to have a contiguous polyp than cancers with BRAF mutation.33 In this study, we observed three traditional serrated adenomas with serrated dysplasia showing sessile serrated adenoma-like lesions in the periphery, and BRAF was mutated in all cases. Whether traditional serrated adenomas were transformed from sessile serrated adenomas remains unclear. However, the traditional serrated adenoma with serrated dysplasia in our collection differs from sessile serrated adenoma by the predominance in the left-sided colon and the lack of potential to develop microsatellite-unstable colon cancer. The occurrence of invasive carcinoma in smaller lesion in traditional serrated adenoma with serrated dysplasia raises clinical concerns regarding the precise classification of traditional serrated adenoma according to the dysplastic pattern exhibited.
When traditional serrated adenoma undergoes nearly complete adenomatous change, they may be undetected in daily routine clinical practice. In our study, tubullovillous adenoma with serrated feature was defined to have an appreciable traditional serrated adenoma component. Tubullovillous adenoma with serrated feature had 10% BRAF mutation rate, had a high frequency of KRAS mutation and positive CpG island methylator phenotype. Nuclear staining of β-catenin was detected in both tubullovillous adenoma with serrated feature and traditional serrated adenoma with conventional adenomatous dysplasia, which showed no marked disparities in their molecular phenotypes, and two serrated adenocarcinomas were in continuity with tubullovillous adenoma with serrated feature. These findings suggest a morphological and molecular spectrum between tubullovillous adenoma with serrated feature and traditional serrated adenoma with conventional adenomatous dysplasia. Another possible interpretation is that tubullovillous adenoma with serrated feature is merely a conventional tubulovillous adenoma with focally serrated changes. However, the positive CpG island methylator phenotype status and BRAF mutation are rarely detected in conventional adenomas.8, 34, 35 Thus, our findings suggest a close association of tubullovillous adenoma with serrated feature with traditional serrated adenoma with conventional adenomatous dysplasia.
In summary, our data indicated that traditional serrated adenoma developed malignancy through two pathways (Figure 4). Traditional serrated adenoma with a KRAS mutation is more likely to develop conventional adenomatous dysplasia and activation of Wnt pathway whereas traditional serrated adenoma with a BRAF mutation usually progresses into serrated dysplasia. Adenocarcinomas arising from traditional serrated adenomas and sessile serrated adenomas displayed distinct pathological and molecular features. Thus, traditional serrated adenoma-associated carcinomas, which account for a certain fraction of colorectal cancers with KRAS or BRAF mutation and colorectal cancers with stable/low levels of microstallite instability, represent a unique serrated pathway of colorectal carcinogenesis.
References
Snover DC, Ahnen DJ, Burt RW et al. Serrated polyps of the colon and rectum and serrated polyposis In: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds). WHO Classification of Tumours of the Digestive System 4th edn. International Agency for Research on Cancer: Lyon, France, 2010;160–165.
Longacre TA, Fenoglio-Preiser CM . Mixed hyperplastic adenomatous polyps/serrated adenomas. Am J Surg Pathol 1990;14:524–537.
Bariol C, Hawkins NJ, Turner JJ et al. Histopathological and clinical evaluation of serrated adenomas of the colon and rectum. Mod Pathol 2003;16:417–423.
Mäkinen MJ, George SM, Jernvall P et al. Colorectal carcinoma associated with serrated adenoma—prevalence, histological features, and prognosis. J Pathol 2001;193:286–294.
Hiyama T, Yokozaki H, Shimamoto F et al. Frequent p53 gene mutations in serrated adenomas of the colorectum. J Pathol 1998;186:131–139.
Torlakovic EE, Gomez JD, Driman DK et al. Sessile serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008;32:21–29.
Kim KM, Lee EJ, Kim YH et al. KRAS mutations in traditional serrated adenomas from Korea herald an aggressive phenotype. Am J Surg Pathol 2010;34:667–675.
O'Brien MJ, Yang S, Mack C et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006;30:1491–1501.
Bettington M, Walker N, Clouston A et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology 2013;62:367–386.
Fu B, Yachida S, Morgan R et al. Clinicopathologic and genetic characterization of traditional serrated adenomas of the colon. Am J Clin Pathol 2012;138:356–366.
Rex DK, Ahnen DJ, Baron JA et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315–1329.
Fu X, Li J, Li K et al. Hypermethylation of APC promoter 1A is associated with moderate activation of Wnt signalling pathway in a subset of colorectal serrated adenomas. Histopathology 2009;55:554–563.
Jass JR, Baker K, Zlobec I et al. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a 'fusion' pathway to colorectal cancer. Histopathology 2006;49:121–131.
García-Solano J, Conesa-Zamora P, Carbonell P et al. Colorectal serrated adenocarcinoma shows a different profile of oncogene mutations, MSI status and DNA repair protein expression compared to conventional and sporadic MSI-H carcinomas. Int J Cancer 2012;131:1790–1799.
Leggett B, Whitehall V . Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010;138:2088–2100.
Jass JR, Whitehall VL, Young J et al. Emerging concepts in colorectal neoplasia. Gastroenterology 2002;123:862–876.
Aust DE, Baretton GB . Serrated polyps of the colon and rectum (hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, and mixed polyps)-proposal for diagnostic criteria. Virchows Arch 2010;457:291–297.
Noffsinger AE . Serrated polyps and colorectal cancer: new pathway to malignancy. Annu Rev Pathol 2009;4:343–364.
Iino H, Jass JR, Simms LA et al. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol 1999;52:5–9.
Schlemper RJ, Riddell RH, Kato Y et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000;47:251–255.
Goldstein NS . Small colonic microsatellite unstable adenocarcinomas and high-grade epithelial dysplasias in sessile serrated adenoma polypectomy specimens: a study of eight cases. Am J Clin Pathol 2006;125:132–145.
Bellizzi AM, Frankel WL . Colorectal cancer due to deficiency in DNA mismatch repair function: a review. Adv Anat Pathol 2009;16:405–417.
Tuppurainen K, Makinen JM, Junttila O et al. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J Pathol 2005;207:285–294.
Weisenberger DJ, Siegmund KD, Campan M et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 2006;38:787–793.
Hall PA, Lane DP . p53 in tumour pathology: can we trust immunohistochemistry?—Revisited!. J Pathol 1994;172:1–4.
Curtin K, Slattery ML, Holubkov R et al. p53 alterations in colon tumors: a comparison of SSCP/sequencing and immunohistochemistry. Appl Immunohistochem Mol Morphol 2004;12:380–386.
Serrano M, Lin AW, McCurrach ME et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997;88:593–602.
Michaloglou C, Vredeveld LC, Soengas MS et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005;436:720–724.
Sheridan TB, Fenton H, Lewin MR et al. Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions ‘caught in the act’. Am J Clin Pathol 2006;126:564–571.
Maltzman T, Knoll K, Martinez ME et al. Ki-ras proto-oncogene mutations in sporadic colorectal adenomas: relationship to histologic and clinical characteristics. Gastroenterology 2001;121:302–309.
Morris RG, Curtis LJ, Romanowski P et al. Ki-ras mutations in adenomas: a characteristic of cancer-bearing colorectal mucosa. J Pathol 1996;180:357–363.
Snover DC . Update on the serrated pathway to colorectal carcinoma.. Hum Pathol 2011;42:1–10.
Rosty C, Young JP, Walsh MD et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod Pathol 2013;26:825–834.
Jass JR . Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007;50:113–130.
Kang GH . Four molecular subtypes of colorectal cancer and their precursor lesions. Arch Pathol Lab Med 2011;135:698–703.
Acknowledgements
We thank the staff of the Second Core Laboratory, Department of Medical Research, National Taiwan University Hospital, for providing technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tsai, JH., Liau, JY., Lin, YL. et al. Traditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesis. Mod Pathol 27, 1375–1385 (2014). https://doi.org/10.1038/modpathol.2014.35
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2014.35
Keywords
This article is cited by
-
Nonconventional dysplasia in patients with inflammatory bowel disease and colorectal carcinoma: a multicenter clinicopathologic study
Modern Pathology (2020)
-
Clinicopathological and molecular correlations in traditional serrated adenoma
Journal of Gastroenterology (2020)
-
Applying Precision to the Management of BRAF-Mutant Metastatic Colorectal Cancer
Targeted Oncology (2020)
-
An update on the morphology and molecular pathology of serrated colorectal polyps and associated carcinomas
Modern Pathology (2019)
-
Superficially serrated adenoma: a proposal for a novel subtype of colorectal serrated lesion
Modern Pathology (2018)