Abstract
Collagenous gastritis is a rare condition defined histologically by a superficial subepithelial collagen layer. This study further characterizes the morphologic spectrum of collagenous gastritis by evaluating a multi-institutional series of 40 patients (26 female and 14 male). The median age at onset was 16 years (range 3–89 years), including 24 patients (60%) under age 18. Twelve patients (30%) had associated celiac disease, collagenous sprue, or collagenous colitis. Hematoxylin and eosin slides were reviewed in biopsies from all patients and tenascin, gastrin, eotaxin, and IgG4/IgG immunohistochemical stains were applied to a subset. The distribution of subepithelial collagen favored the body/fundus in pediatric patients and the antrum in adults. There were increased surface intraepithelial lymphocytes (>25 lymphocytes/100 epithelial cells) in five patients. Three of these patients had associated celiac and/or collagenous sprue/colitis, while the remaining two had increased duodenal lymphocytosis without specific etiology. An eosinophil-rich pattern (>30 eosinophils/high power field) was seen in 21/40 (52%) patients. Seven patients’ biopsies demonstrated atrophy of the gastric corpus mucosa. Tenascin immunohistochemistry highlighted the subepithelial collagen in all 21 specimens evaluated and was a more sensitive method of collagen detection in biopsies from two patients with subtle subepithelial collagen. No increased eotaxin expression was identified in 16 specimens evaluated. One of the twenty-three biopsies tested had increased IgG4-positive cells (100/high power field) with an IgG4/IgG ratio of 55%. In summary, collagenous gastritis presents three distinct histologic patterns including a lymphocytic gastritis-like pattern, an eosinophil-rich pattern, and an atrophic pattern. Eotaxin and IgG4 were not elevated enough to implicate these pathways in the pathogenesis. Tenascin immunohistochemistry can be used as a sensitive method of collagen detection.
Similar content being viewed by others
Main
Collagenous gastritis is a rare form of gastritis defined histologically by the presence of >10 μm of subepithelial surface collagen deposition with an associated inflammatory infiltrate in the lamina propria. The condition was first described in 1989 by Colletti and Trainer.1, 2 To date, ∼50 patients with collagenous gastritis have been reported in the English language literature, mainly as reports of one or two cases.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The two largest previous series comprised 6 and 12 patients, respectively.16, 17
On the basis of patient age and clinical presentation, Lagorce-Pages et al16 recognized two general clinical subgroups in collagenous gastritis. The first group included children with isolated collagenous gastritis, a nodular stomach on gastroscopy, and iron deficiency anemia, hypothesized to be due to bleeding from superficial capillaries entrapped in collagen.6, 18 The second group consisted of adults presenting with chronic watery diarrhea and displaying associated collagenous colitis. While a significant proportion of patients with collagenous gastritis will fall into one of these two categories, a number of reported patients do not easily fit in one of these categories. Histologically, while subepithelial collagen and an associated inflammatory infiltrate are universally present, the inflammatory pattern varies considerably in biopsies from different patients.
Although an association with autoimmune diseases, particularly celiac sprue, has been noted, the pathogenesis of collagenous gastritis remains uncertain. The different age groups and presentations raise the possibility that more than one etiologic mechanism may be responsible. Increased stromal collagen deposition occurs in other disorders of the digestive system, including eosinophilic espohagitis and IgG4-related disease, in particular autoimmune pancreatitis. Eosinophilic esophagitis has been linked to allergy and the chemokine eotaxin has been implicated in the pathogenesis.19 While it remains unclear whether IgG4 itself is pathogenic or a side effect in IgG4-related disease, increased levels of this class of immunoglobulin in serum and tissue plasma cells are a defining feature in IgG4-related disease.20
Through detailed evaluation of biopsies from 40 patients, the present study aims to characterize distinct inflammatory patterns in collagenous gastritis and determine whether any of these variants are associated with particular clinical subgroups defined by factors such as age at presentation, disease association, or long-term outcome. Possible pathogenic mechanisms related to IgG4 and eotaxin are also investigated. Finally, the value of tenascin immunohistochemistry, which has been shown to be a useful diagnostic adjunct in collagenous colitis,21 is assessed as well.
Materials and methods
Patient Identification and Histologic Review
Pathology records were searched to identify all pathologic diagnoses of collagenous gastritis from routine surgical pathology and consultation practice at five institutions (Massacusetts General Hospital, Boston, MA, USA; Boston Children’s Hospital, Boston MA, USA; Royal Brisbane and Women’s Hospital, Brisbane, Qld, Australia; Envoi Pathology, Brisbane, Qld, Australia, and Sullivan Nicolaides Pathology, Brisbane, Qld, Australia). This included a set of 10 pediatric patients from outside hospitals that were reviewed in consultation at the Massachusetts General Hospital for a related clinical study. Hematoxylin and eosin (H&E) slides from the initial diagnostic biopsies and all available follow-up biopsies were obtained and reviewed. All specimens were formalin fixed, paraffin embedded, and sectioned at 4–5 μm. At least one gastric biopsy was required to display a subepithelial collagen deposition of >10 μm in thickness. Additional features reviewed systematically in both the initial and all subsequent follow-up gastric biopsies included: maximum collagen thickness in μm (measured by ocular micrometer), entrapment of capillaries by collagen, denudation (sloughing) of surface epithelium (categorized as absent, <30% of involved surface, >30% of involved surface), extent of collagen distribution (categorized as ‘focal’ if <30% of surface, ‘patchy’ if 30–69% of surface, and ‘diffuse’ if ≥70% of surface was affected), affected mucosa (antral predominant, corpus predominant, or approximately equal in both regions) and the presence or absence of intestinal metaplasia. The severity of chronic inflammation (mild and moderate) was judged with guidance from the visual analogs provided by the updated Sydney system.22 For active inflammation, we applied the term ‘focal’ active inflammation to biopsies with a single focus of stromal neutrophilic inflammation, with rare, if any, associated intraepithelial neutrophils. ‘Mild’ active inflammation was defined by more than one such focus and ‘moderate’ active inflammation by the presence of multifocal neutrophilic inflammation within the glands and pits. Gastric atrophy was identified by an apparent decrease in the number of gland profiles in the oxyntic mucosa with marked reduction in the specialized cells lining the oxyntic glands (including parietal and chief cells), pyloric metaplasia, and splaying of hyperplastic smooth muscle in the lamina propria. The maximum number of intraepithelial lymphocytes per 100 surface epithelial cells and maximum number of eosinophils per high power field of lamina propria ( × 400 magnification) were recorded. A threshold of >30 eosinophils per × 400 high power field was considered as significant, as this number has been previously used to define ‘histological eosinophilic gastritis’.23 The biopsies were also assessed for the presence of Helicobacter pylori and, when available, histochemical and immunohistochemical stains were also reviewed. All available follow-up biopsies were assessed for histologic evidence of disease resolution or persistence. Concurrent and follow-up mucosal biopsies from other sites in the gastrointestinal tract were reviewed and any diagnostic abnormalities were recorded.
Medical Record Review
All surgical pathology reports and available clinical records were reviewed to determine age at diagnosis, gender, presenting symptoms, the presence of anemia at the time of diagnosis, results of H. pylori and celiac serology testing. A history of celiac sprue, collagenous sprue, collagenous colitis, eosinophilic esophagitis, and IgG4-related disease were searched for in each patient. In addition to searching for any specific mention of IgG4-related disease, we searched for diseases potentially related to IgG4 including autoimmune pancreatitis, sclerosing cholangitis, Mikulicz’s syndrome, Küttner’s tumor, Riedel’s thyroiditis, orbital pseudotumor, orbital myositis, tubulointerstitial nephritis, aortitis/periaortitis, retroperitoneal fibrosis, mediastinal fibrosis, and multifocal fibrosclerosis. Other chronic medical conditions and any history of autoimmune disease were recorded. In patients with biopsies that had no residual collagen at last follow-up, medical records (when available) were reviewed to determine whether there was symptom resolution.
Immunohistochemistry
Immunohistochemical evaluation of IgG, IgG4, synaptophysin, gastrin, and tenascin were performed on 4 μm thick sections using a Leica Bond III automated slide stainer (Leica, Buffalo Grove, IL, USA) and Leica Bond Polymer DAB Detection kits (Table 1). Palatine tonsil tissue served as a positive control for IgG and IgG4. In each available biopsy, the maximum number of IgG4-positive cells per high power field was counted in the area of greatest density in the lamina propria and the ratio of IgG4-positive cells/IgG-positive cells in that area was counted. G-cells and Enterochromaffin-like (ECL) cells in normal gastric mucosa served as controls for gastrin and synaptophysin. The absence of gastrin-positive cells was used to confirm biopsy location in the gastric oxyntic mucosa and exclude antral location. For tenascin, sections from a patient with established collagenous colitis served as the positive control tissue and normal gastric mucosa (uninvolved by collagenous gastritis) served as a negative control. Subepithelial collagen staining in the gastric mucosa, similar to the pattern in collagenous colitis, was considered to be a positive result.
Immunohistochemistry for eotaxin was performed manually. Four micron sections were deparaffinized and antigen retrieval was performed using a Citrate buffer decloaker, followed by a 5-min incubation with dual endogenous enzyme block (Dako, Glostrup, Denmark) at room temperature. The eotaxin antibody (ab133604; Abcam, Cambridge, MA, USA) was diluted 1:250 in SignalStain diluent (Cell Signalling, Danvers, MA, USA) and incubated overnight at 4 °C. The sections were also incubated with SignalStain Boost IHC Detection Reagent (Cell Signalling) for 30 min, followed by a 10-min incubation with DAB+ (Dako). Between each step, the slides were rinsed in Tris-Buffered Saline with Tween-20. Sections from inflammatory nasal polyps were used as a positive control with staining of the inflammatory cell infiltrate and epithelium interpreted as immunopositivity for eotaxin. In the gastric mucosa (normal stomach controls and collagenous gastritis), we assessed staining for eotaxin in the same locations, the inflammatory cell infiltrate, and the epithelium.
Results
Clinical Characteristics of the Study Population and Disease Associations
Forty patients, including 26 females (65%) and 14 males (35%) made up the study group. The median age at onset was 16 years, with a range 3–89 years. Most of the patients (24/40=60%) presented at 18 years of age or less. Clinical characteristics of the cohort with outcomes are summarized in Table 2. Information about the clinical presentation was available in 38 patients, half of which had more than one significant sign or symptom. The pediatric population most commonly presented with abdominal pain (57%), anemia (39%), nausea/vomiting (26%), or a documented gastrointestinal bleed with hematemesis or melena stool (22%). In adults, anemia was the most common presenting sign (67%), followed by diarrhea (27%) and abdominal pain (27%). Collagenous gastritis was diagnosed incidentally to other pathology in one patient. We had a description of the endoscopic appearance in 29 patients, which most often revealed a nodular stomach (76% of patients). Three patients, two of which were under 18 years of age, had associated celiac sprue (confirmed by histology and serology). The adult celiac patient also had collagenous sprue and collagenous colitis. There were four additional patients (three pediatric and one adult) with normal villous architecture and a significant intraepithelial lymphocytosis (>30 per 100 enterocytes in the villous tips) on duodenal biopsy. In one of these patients, there was a documented negative Tissue Transglutaminase and in three of these patients we did not have results of serologic tests for celiac sprue. There was also a pediatric patient with a documented positive Tissue Transglutaminase test, but normal duodenal histology. Associated collagenous sprue and collagenous colitis was more common in adults than in the pediatric population (P<0.05), affecting 7 of the 16 adults (44%) and only 2 patients in the pediatric cohort. Significant co-morbidities identified in the pediatric cohort included one patient each with type 1 diabetes mellitus, juvenile arthritis, asthma, eczema, genetic coagulopathy (Factor VII deficiency), and migraine headaches. One child was noted to be below the 5th percentile for height and weight. In the adult cohort, comorbidities included liver cirrhosis (related to hepatitis C and alcohol), Graves disease, rheumatoid arthritis, epilepsy, and coronary artery disease in one patient each. There were two patients with diabetes mellitus in the adult cohort, one of which was type II diabetes and the other undetermined based on the available history. We did not have significant background medical history for review in 13 of the 40 patients (32%).
Histology and Inflammatory Patterns
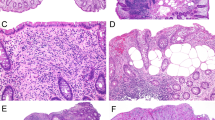
The pathologic findings in the 40 patients are summarized in Table 3. The subepithelial collagen deposition in the initial biopsy ranged in maximum thickness from 15 to 115 μm (mean 55.1 μm; 95% confidence interval (CI): 45–65 μm). Where biopsies of both gastric corpus and antrum were taken, the collagen deposition was corpus predominant in 55% (22/40) of patients. This corpus predominant distribution was more common (P<0.05) in the pediatric patients (71%) than in adults (31%). In nine cases, only the corpus was involved by collagen and the antrum was completely spared and in two cases only the antrum was involved and the corpus was completely spared. Inflammatory expansion of the lamina propria was present in all patients. Usually, this was mild or moderate in degree, and was judged to be of moderate severity in the adult population (75%) slightly more often (P=0.05) than in the pediatric population (42%). Active (neutrophilic) inflammation was identified in half of the patients and was usually focal or mild. Intraepithelial lymphocytes ranged from 5 to 45 per 100 surface epithelial cells. In 5 patients (12%), the intraepithelial lymphocyte density was >25 intraepithelial lymphocytes/100 surface epithelial cells, which is the threshold that is used to define lymphocytic gastritis (eg, Figure 1).24 Often the intraepithelial lymphocytes were most increased in areas of the gastric biopsy displaying lesser degrees of collagen deposition. Two of these five patients had associated celiac sprue and one had collagenous sprue in the duodenum as well as collagenous colitis but no confirmed celiac. The remaining two patients did have an increase in intraepithelial lymphocytes in the duodenum, with preserved villous architecture, one with negative Tissue Transglutaminase and the other no available celiac serology testing. Three of the five patients with increased gastric intraepithelial lymphocytes had collagenous colitis. An increased eosinophilic infiltrate of the lamina propria (eg, Figure 2) was noted in all biopsies with a mean count in the area of greatest eosinophil density of 41 eosinophils (95% CI: 30–53) in the pediatric biopsies compared with 29 (95% CI: 19–40) in the adult population. There was a trend toward more pediatric biopsies exceeding a threshold of at least 30 eosinophils per high power field (62%) compared with adults (38%), but this was not statistically significant. This was partly related to the presence of high eosinophil counts in young adults under age 40. We noted that only 2 of 9 patients over age 35 had an eosinophil count of above 30 per high power field.
(a) Collagenous gastritis with prominent intraepithelial lymphocytosis (>25 per 100 surface epithelial cells) above the collagen table. (b) The increased intraepithelial lymphocytosis is more prominent in areas of gastric mucosa uninvolved by collagen. (c) The duodenal biopsies from the same patient show villous blunting and intraepithelial lymphocytosis compatible with celiac sprue.
(a) Biopsy of collagenous gastritis with prominent mucosal eosinophils, numbering over 100 per × 400 high power field. (b) Another patient’s biopsies with prominent mucosal eosinophils, numbering 80 per × 400 high power field. Eosinophils in the lamina propria are shown inset at higher magnification. (c) The same section as illustrated in ‘b’ was negative for eotaxin by immunohistochemistry. Positive eotaxin staining in a control inflammatory sinonasal polyp is shown as inset.
Four of the biopsies (all from adults) displayed intestinal metaplasia in the gastric mucosa on initial biopsy or on follow-up. Seven biopsies (5 pediatric and 2 adults aged 24 and 44 years) displayed moderate to severe atrophy of the gastric corpus mucosa with associated smooth muscle hyperplasia in the lamina propria and pseudopyloric metaplasia (eg, Figure 3). The location of the atrophic mucosa in the gastric corpus was confirmed by gastrin immunostaining in six biopsies. For the seventh biopsy the interpretation was based on reported biopsy location in the corpus without a confirmatory immunostain (unavailable tissue block). Two of these seven biopsies with atrophic corpus mucosa also displayed intestinal metaplasia. Of note, there was minimal ECL cell hyperplasia in these biopsies based on synaptophysin staining. H. pylori infection was identified in tissue in only one patient, a 45-year-old male. In the remaining patients, the absence of H. pylori infection diagnosed on H&E was also confirmed by immunohistochemistry (23 patients), by Giemsa/Diff-Quick (5 additional patients), and by fecal PCR in 1 patient.
(a) Biopsy of gastric body mucosa involved by collagenous gastritis with moderate to severe atrophy of the oxyntic glands, including a reduction in the number of glandular profiles and loss of the specialized cells (parietal and chief cells) of the oxyntic glands. The associated smooth muscle hyperplasia in the lamina propria is prominent. The subepithelial collagen band is highlighted with arrowheads. (b) Another biopsy fragment of corpus mucosa from the same patient demonstrates oxyntic gland atrophy with pyloric metaplasia. Note the splayed surface collagen with capillary entrapment and a denuded epithelial surface. (c) A gastrin immunohistochemical stain from the same area as ‘b’ shows that no G cells are present, confirming the location of this atrophic mucosa is the gastric corpus.
Table 4 summarizes the clinical characteristics of the patients with each of three inflammatory patterns: a lymphocytic gastritis-like pattern, an eosinophil-rich pattern, and an atrophic pattern. There were no patients with both an increase in intraepithelial lymphocytes and atrophy of the corpus mucosa. However, all seven patients with atrophy of the corpus mucosa had increased eosinophils in the lamina propria (>30 per high power field) and two of the five patients with increased intraepithelial lymphocytes had a concurrent increase in lamina propria eosinophils exceeding that threshold.
Follow-up
Follow-up biopsies were available in 23 (58%) patients. There was a median follow-up time of 1.9 years (mean 2.4 years). Eighteen of these patients showed persistence of the subepithelial collagen band, lasting up to 10 years from initial biopsy. Of the five patients who had no collagen at last biopsy, three were pediatric and two were adults. The biopsy with no residual collagen was taken at a mean of 3 years (range 0.8–6 years) after initial presentation. One of the adults had associated collagenous sprue and collagenous colitis. Four patients with no residual collagen at last biopsy had >30 eosinophils per high power field at initial biopsy and one was a pediatric patient with moderate gastric atrophy. At least one of these patients had symptom resolution, but we had incomplete clinical follow-up data documenting persistence or resolution of symptoms in most patients.
Immunohistochemistry for Eotaxin and IgG4
Immunohistochemical staining for eotaxin was applied to 16 biopsies. None demonstrated significant eotaxin immunopositivity in a similar pattern to the control inflammatory sinonasal polyps. Figure 2 shows the biopsy from a patient with collagenous gastritis with an eosinophil-rich inflammatory pattern and negative immunohistochemical staining for eotaxin. A combination of IgG4 and IgG immunohistochemical stains was performed in 23 biopsies. One of these showed a significant increase in IgG4-positive plasma cells, with up to 100 positive cells per high power field and an IgG4/IgG ratio of 55%. In all sections tested, the median IgG4-positive plasma cell count was 10 per high power field (mean 18, 95% CI: 9.7–26). The median IgG4/IgG ratio was 12 (mean 17.6, 95% CI: 12–23). The single patient with an elevated IgG4/IgG ratio and all other patients with medical history available for review had no history of IgG4-related disease. Of note, we had a detailed past medical history for review in only 27 of 40 patients.
Immunohistochemistry for Tenascin
An immunohistochemical stain for tenascin was applied to biopsies from 21 patients, all of which demonstrated immunopositivity in the subepithelial collagen band that matched the corresponding H&E or trichrome slide. An example is illustrated in Figure 4. The tenascin stain had a moderate to strong intensity in 18 biopsies and weak intensity in 3 biopsies. The tenascin stain spared the subepithelial location in background gastric mucosa uninvolved by collagen on H&E/trichrome in the biopsies from all but two patients. In one of these patients, the tenascin highlighted a thin subepithelial layer of presumable collagen in a fragment of background mucosa that was uninvolved by collagen on H&E and trichrome. The second patient of interest had a repeat biopsy following a biopsy diagnosis of collagenous gastritis (reviewed and confirmed) taken 10 months earlier. This follow-up biopsy was reported to be uninvolved by subepithelial collagen and our review of the H&E and trichrome confirmed this impression. However, the tenascin stain did reveal a thin subepithelial collagen layer that was imperceptible with histochemical stains alone. This patient’s biopsy is illustrated in Figure 5. Of note, this patient’s symptoms (epigastric pain) were persistent at this follow-up biopsy and 3 years after the follow-up biopsy.
(a) Collagenous gastritis highlighted by trichrome in a biopsy involved by collagen at left and one uninvolved by collagen at right. (b) Tenascin immunohistochemistry of the same as in ‘a’ shows strong immunopositivity in the area with subepithelial collagen, with sparing of the biopsy uninvolved. Note that the muscularis mucosa is also tenascin positive. (c) A high power view of the trichrome stain of the biopsy in ‘a’ with subepithelial collagen. (d) The same area is highlighted more intensely and with a thicker distribution using tenascin immunohistochemistry.
(a) In this patient’s biopsies, subepithelial collagen was readily identified on the initial set of H&E stained biopsies, with associated sloughing of the surface epithelium. (b) Tenascin staining in the same area highlights the collagen deposition. (c) A follow-up biopsy from the same patient 10 months later was initially reported as negative for subepithelial collagen. (d) Application of a tenascin stain revealed a thin layer of persistent thickened subepithelial collagen not detectable by histochemical stains.
Tenascin immunostaining was negative in the control normal gastric mucosa tested. The smooth muscle of the muscularis propria was also highlighted by tenascin, which was particularly prominent in collagenous gastritis biopsies from three patients with atrophy of the corpus mucosa and smooth muscle hyperplasia. This phenomenon was easily distinguishable from collagen due to the basal rather than subepithelial location of smooth muscle.
Discussion
Due to the rarity of collagenous gastritis, it is difficult for investigators to evaluate a large series of patients. The present work resulted from collaboration between five institutions and included a significant volume of consultations from outside hospitals, which enabled us to review a large cohort. This allowed us to appreciate the histologic spectrum of collagenous gastritis. Biopsies from all patients share the defining features of subepithelial collagen deposition and inflammation in the lamina propria. However, there is marked heterogeneity in the associated inflammatory pattern. Collagenous gastritis occurs in three distinctive inflammatory milieus, a lymphocytic gastritis-like pattern, an eosinophil-rich pattern, and an atrophic gastritis pattern. To define the lymphocytic gastritis-like pattern, we applied the same criteria which define lymphocytic gastritis, typically cited as at least 25 intraepithelial lymphocytes per high power field.24 Outside the setting of collagenous gastritis, lymphocytic gastritis has been frequently associated with celiac sprue, H. pylori infection, and more rarely with Crohn’s disease, syphilis infection, and Menetrier’s disease.24 Only one patient in our study had concurrent collagenous gastritis and H. pylori infection, and this patient had no significant increase in intraepithelial lymphocytes. Two of the patients with a lymphocytic gastritis-like inflammatory pattern had celiac, one had collagenous sprue in the duodenum, and the remaining two had increased intraepithelial lymphocytes in the duodenum. That histologic finding raises the possibility that the latter two patients could have a latent form of celiac sprue. Previous case reports of collagenous gastritis have noted a lymphocytic gastritis-like inflammatory pattern and noted an association with celiac sprue.4, 5 In a previous report, Leung et al17 noted that 4 of their 12 patients had lymphocytic gastritis, two of which had associated celiac disease or collagenous colitis.
In defining the eosinophil-rich pattern of collagenous gastritis, we used a similar threshold (>30 eosinophils per × 400 high power field) that has been used to define histologic eosinophilic gastritis.23 Collagenous gastritis is a part of the differential diagnosis for a histologic finding of eosinophilic gastritis and the eosinophilia should prompt a careful search for subepithelial collagen. There seems to be a trend toward increased eosinophils in younger patients, but it is important to underscore that all patients with collagenous gastritis we have observed have at least a minor degree of eosinophilic inflammation. Notably, 3 of 12 patients (25%) in Leung’s series were noted to have ‘prominent’ eosinophils, and these included two of three pediatric patients in a predominantly adult series. There are also reports of collagenous gastritis in adolescents and young adults that have made a particular note of prominent lamina propria eosinophilia and raised the possibility that the collagen deposition may be due to an immune mechanism related to tissue eosinophilia.14, 25
The atrophic pattern is a relatively uncommon but very distinctive pattern in our series. The atrophic pattern is characterized by atrophy of the oxyntic mucosa with marked reduction in the specialized cells lining the oxyntic glands (including parietal and chief cells), pyloric metaplasia, and splaying of hyperplastic smooth muscle in the lamina propria. Recognition of the biopsy location in the corpus is very important in identifying this pattern, which can be mistaken histologically for biopsy of an antral or pyloric location. Negative gastrin immunohistochemical staining is helpful to confirm the corporal biopsy location and support the H&E interpretation of atrophy. The atrophic pattern bears some resemblance to the atrophic pattern of the corpus mucosa noted in autoimmune gastritis. Furthermore, two of these patients with ‘atrophic’ collagenous gastritis also showed intestinal metaplasia. The lack of intestinal metaplasia in the other patients may be partly related to a relatively short follow-up period. The atrophic pattern has not been a well-established inflammatory pattern in prior studies. Leung et al17 identified no atrophy in their patients despite a systematic assessment for this feature. A 12-year follow-up from the 1989 case report by Colletti and Trainer noted moderate glandular atrophy.2 However, while they noted endocrine cell hyperplasia, this was not apparent in our series.
Clinically, we are able to confirm that there is a female disease predominance and a significant number of presentations in adolescents and young adults. The cohort we present here has a selection bias that favors pediatric patients due to the inclusion of a pediatric hospital and a significant number of pediatric consultations that were reviewed for a related study. Clinically, symptomatic anemia and abdominal pain were the most common presenting symptoms. The cause of these symptoms is not fully understood, but some authors have speculated that the anemia is due to bleeding from superficial dilated capillaries entrapped by collagen.6, 16 There is limited evidence in H. pylori gastritis that the pathogenesis of dyspepsia may be related to chronic inflammation.26 Whether an inflammatory mediated or other mechanism causes the dyspepsia of collagenous gastritis remains unclear. The classic nodular endoscopic picture was quite common in this cohort. Our finding of antral predominant disease in adults and corpus predominant disease in children is interesting and may be a helpful point for endoscopists to be aware of to guide sampling. Since a significant proportion of patients have collagen involving only the antrum or corpus (more than 25% this study), both locations should be sampled by endoscopists considering the diagnosis.
The long-term outcome for collagenous gastritis remains uncertain. Our series indicates that the process persists for years histologically in the majority of patients. Although we had incomplete follow-up, the absence of collagen at final follow-up biopsy in five patients, including one with confirmed symptom resolution, indicates that the disease will occasionally resolve fully. Given the patchy nature of the collagen distribution in biopsy specimens, it should be noted that the absence of collagen on follow-up biopsies may be due to sampling error rather than actual disease resolution.
The etiology of collagenous gastritis remains unclear and the pathophysiology poorly understood. We investigated two possible mechanisms, related to eotaxin and IgG4. Eotaxin has been implicated previously in the pathogenesis of eosinophilic esophagitis.19, 27 Fibrosis with subepithelial collagen deposition is also a feature of eosinophilic esophagitis.28 The prominent lamina propria eosinophilia in many of our patients’ biopsies was further rationale for testing the tissue for eotaxin. The absence of appreciably increased eotaxin staining means we are unable to implicate increased eotaxin levels in the pathogenesis of collagenous gastritis. However, our results are limited by the sensitivity of the method we used. Immunohistochemistry may detect only very elevated levels of eotaxin, as in the control inflammatory nasal polyps we used. However, some eosinophilic esophagitis studies have applied a more sensitive quantitative real-time PCR protocol for detection of increased tissue eotaxin levels.19 This type of study would be required before a role for eotaxin can be completely excluded.
IgG4-related diseases have been recently recognized in a number of inflammatory conditions involving many sites throughout the body.20 The prototypical IgG4-related disease, autoimmune pancreatitis, includes storiform fibrosis as a defining histologic feature. Other IgG4-related diseases such as retroperitoneal fibrosis and Riedel’s thyroiditis are associated with significant collagen deposition. Reported gastric manifestations of IgG4-related disease have included a diffuse increase in IgG4-positive plasma cells with or without chronic ulcers, without associated subepithelial collagen.29, 30, 31, 32 We did identify a significant number of IgG4-positive plasma cells (100 cells per HPF; IgG4/IgG ratio of 55%) in 1 biopsy out of 23 tested. Although this patient’s biopsy exceeded the IgG4/IgG ratio of 50% reportedly diagnostic of IgG4-related disease,20 we felt that this finding was too infrequent to implicate IgG4 in the pathogenesis of collagenous gastritis. Further, we did not identify a history of systemic IgG4-related disease in this or any other patient.
Tenascin immunohistochemistry has been proposed to be a useful ancillary test to support the histologic diagnosis of collagenous colitis, with sensitivity superior to H&E and trichrome stains.21 However, before this study, tenascin expression has not been assessed in collagenous gastritis. Tenascin staining did confirm the presence of subepithelial collagen in all biopsies we tested. The two biopsies in which we identified subepithelial tenascin staining in areas where collagen was imperceptible on H&E (eg, Figure 5) raise the possibility that tenascin may have a role in detecting cases of collagenous gastritis that are too subtle to detect with histochemical stains alone, although further study would be required to establish the hypothesis that tenascin is a more sensitive marker than trichrome in this setting. Given the patchy distribution of collagen and thin collagen band in some biopsies, the possibility of using a tenascin stain to detect subtle cases of collagenous gastritis (perhaps including cases with a collagen band less than the current definitional 10 μm thickness) in patients with a suspicious clinical picture and background inflammatory pattern is an intriguing possible direction for future study. While tenascin may have a role in future research and a limited role as a sensitive marker to identify diagnostically challenging cases of collagenous gastritis, it is important to recognize that tenascin is generally not required to make the diagnosis. The collagen in all 40 patients studied here was originally identified with histochemical stains alone.
In conclusion, this large series of patients with collagenous gastritis supports a female disease predominance. In addition to the diagnostic irregular subepithelial collagen deposition, three distinct inflammatory patterns can be seen including a lymphocytic gastritis-like pattern, an eosinophil-rich pattern, and an atrophic pattern. Awareness of the association of these three inflammatory patterns with collagenous gastritis may prompt pathologists to look carefully for collagen in subtle cases. Collagen often persists for years, but may resolve histologically in a minority of patients. The etiology of collagenous gastritis remains uncertain, but our investigations seem to exclude a mechanism related to increased eotaxin or IgG4. Finally, tenascin immunohistochemisrty appears to be a sensitive marker for the disease and is an area for future investigation in this disorder.
References
Colletti RB, Trainer TD . Collagenous gastritis. Gastroenterology 1989;97:1552–1555.
Winslow JL, Trainer TD, Colletti RB . Collagenous gastritis: a long-term follow-up with the development of endocrine cell hyperplasia, intestinal metaplasia, and epithelial changes indeterminate for dysplasia. Am J Clin Pathol 2001;116:753–758.
Freeman H, Piercey J, Raine R . Collagenous gastritis. Can J Gastroenterol 1989;3:171–174.
Stancu M, De Petris G, Palumbo TP et al. Collagenous gastritis associated with lymphocytic gastritis and celiac disease. Arch Pathol Lab Med 2001;125:1579–1584.
Vesoulis Z, Lozanski G, Ravichandran P et al. Collagenous gastritis: a case report, morphologic evaluation, and review. Mod Pathol 2000;13:591–596.
Cote JF, Hankard GF, Faure C et al. Collagenous gastritis revealed by severe anemia in a child. Hum Pathol 1998;29:883–886.
Groisman GM, Meyers S, Harpaz N . Collagenous gastritis associated with lymphocytic colitis. J Clin Gastroenterol 1996;22:134–137.
Castellano VM, Munoz MT, Colina F et al. Collagenous gastrobulbitis and collagenous colitis. Case report and review of the literature. Scand J Gastroenterol 1999;34:632–638.
Pulimood AB, Ramakrishna BS, Mathan MM . Collagenous gastritis and collagenous colitis: a report with sequential histological and ultrastructural findings. Gut 1999;44:881–885.
Meunier S, Villard F, Bouvier R et al. [Collagen gastritis, an unusual cause of anemia in children. Report of 2 cases]. Arch Pediatr 2001;8:47–50.
Freeman HJ . Topographic mapping of collagenous gastritis. Can J Gastroenterol 2001;15:475–478.
Dray X, Reignier S, Vahedi K et al. Collagenous gastritis. Endoscopy 2007;39 (Suppl 1):E292–E293.
Kamimura K, Kobayashi M, Narisawa R et al. Collagenous gastritis: endoscopic and pathologic evaluation of the nodularity of gastric mucosa. Dig Dis Sci 2007;52:995–1000.
Park S, Kim DH, Choe YH et al. Collagenous gastritis in a Korean child: a case report. J Korean Med Sci 2005;20:146–149.
Ravikumara M, Ramani P, Spray CH . Collagenous gastritis: a case report and review. Eur J Pediatr 2007;166:769–773.
Lagorce-Pages C, Fabiani B, Bouvier R et al. Collagenous gastritis: a report of six cases. Am J Surg Pathol 2001;25:1174–1179.
Leung ST, Chandan VS, Murray JA et al. Collagenous gastritis: histopathologic features and association with other gastrointestinal diseases. Am J Surg Pathol 2009;33:788–798.
Wilson C, Thompson K, Hunter C . Nodular collagenous gastritis. J Pediatr Gastroenterol Nutr 2009;49:157.
Bhattacharya B, Carlsten J, Sabo E et al. Increased expression of eotaxin-3 distinguishes between eosinophilic esophagitis and gastroesophageal reflux disease. Hum Pathol 2007;38:1744–1753.
Stone JH, Zen Y, Deshpande V . IgG4-related disease. N Engl J Med 2012;366:539–551.
Muller S, Neureiter D, Stolte M et al. Tenascin: a sensitive and specific diagnostic marker of minimal collagenous colitis. Virchows Arch 2001;438:435–441.
Dixon MF, Genta RM, Yardley JH et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–1181.
Lwin T, Melton SD, Genta RM . Eosinophilic gastritis: histopathological characterization and quantification of the normal gastric eosinophil content. Mod Pathol 2011;24:556–563.
Carmack SW, Lash RH, Gulizia JM et al. Lymphocytic disorders of the gastrointestinal tract: a review for the practicing pathologist. Adv Anat Pathol 2009;16:290–306.
Kajino Y, Kushima R, Koyama S et al. Collagenous gastritis in a young Japanese woman. Pathol Int 2003;53:174–178.
Suzuki H, Moayyedi P . Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013;10:168–174.
Tantibhaedhyangkul U, Tatevian N, Gilger MA et al. Increased esophageal regulatory T cells and eosinophil characteristics in children with eosinophilic esophagitis and gastroesophageal reflux disease. Ann Clin Lab Sci 2009;39:99–107.
Lucendo AJ, Arias A, De Rezende LC et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. J Allergy Clin Immunol 2011;128:1037–1046.
Bateman AC, Sommerlad M, Underwood TJ . Chronic gastric ulceration: a novel manifestation of IgG4-related disease? J Clin Pathol 2012;65:569–570.
Fujita T, Ando T, Sakakibara M et al. Refractory gastric ulcer with abundant IgG4-positive plasma cell infiltration: a case report. World J Gastroenterol 2010;16:2183–2186.
Shinji A, Sano K, Hamano H et al. Autoimmune pancreatitis is closely associated with gastric ulcer presenting with abundant IgG4-bearing plasma cell infiltration. Gastrointest Endosc 2004;59:506–511.
Uehara T, Hamano H, Kawa S et al. Chronic gastritis in the setting of autoimmune pancreatitis. Am J Surg Pathol 2010;34:1241–1249.
Acknowledgements
Dr Arnason’s fellowship funding was provided through scholarships from the Royal College of Physicians and Surgeons of Canada (Detweiler Traveling Fellowship) and Dalhousie University (McLoughlin Scholarship). Dr Harland Winter’s work on collagenous gastritis was supported by a philanthropic grant from Martin Schlaff.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Winter discloses Board membership or consultancy work for Janssen Pharmaceuticals, Pediatric IBD Foundation, Prometheus, Salix, AstraZeneca, Nutricia, Mead Johnson Nutritional Division, Parexel, and Shire. Dr Goldsmith declares consultancy work for Cohera Medical.
Rights and permissions
About this article
Cite this article
Arnason, T., Brown, I., Goldsmith, J. et al. Collagenous gastritis: a morphologic and immunohistochemical study of 40 patients. Mod Pathol 28, 533–544 (2015). https://doi.org/10.1038/modpathol.2014.119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2014.119
This article is cited by
-
Collagenous Gastritis Is an Underdiagnosed Cause of Anemia and Abdominal Pain: Systematic Scoping Review
Digestive Diseases and Sciences (2023)
-
Mixed lymphocytic and collagenous inflammation of the entire gastrointestinal tract under therapy with serotonin and norepinephrine reuptake inhibitors
Virchows Archiv (2022)
-
The differential diagnosis of Helicobacter pylori negative gastritis
Virchows Archiv (2018)
-
Diagnosis and treatment of microscopic colitis
Clinical Journal of Gastroenterology (2016)