Abstract
Angiosarcomas constitute a heterogeneous group of highly malignant vascular tumors. Angiosarcoma of bone is rare and poorly characterized. For angiosarcoma of soft tissue, some pathways seem to be involved in tumor development. Our aim was to evaluate the role of these pathways in angiosarcoma of bone. We collected 37 primary angiosarcomas of bone and used 20 angiosarcomas of soft tissue for comparison. Immunohistochemistry was performed on constructed tissue microarrays to evaluate expression of CDKN2A, TP53, PTEN, BCL2, CDK4, MDM2, cyclin D1, β-catenin, transforming growth factor-β (TGF-β), CD105, phospho-Smad1, phospho-Smad2, hypoxia-inducible factor-1α, plasminogen activator inhibitor type 1 (PAI-1), VEGF, CD117 and glucose transporter--1. PIK3CA was screened for hotspot mutations in 19 angiosarcomas. In nearly 55% of the angiosarcoma of bone, the retinoblastoma (Rb) pathway was affected. Loss of CDKN2A expression was associated with a significantly worse prognosis. No overexpression of TP53 or MDM2 was found, suggesting that the TP53 pathway is not important in angiosarcoma of bone. Angiosarcoma of bone showed highly active TGF-β signaling with immunoreactivity for phospho-Smad2 and PAI-1. Although the phosphatidylinositol 3-kinase (PI3K)/Akt pathway seems to be active in both tumor groups, different mechanisms were involved: 41% of angiosarcoma of bone showed a decrease in expression of PTEN, whereas in angiosarcoma of soft tissue overexpression of KIT was found (90%). PIK3CA hotspot mutations were absent. In conclusion, the Rb pathway is involved in tumorigenesis of angiosarcoma of bone. The PI3K/Akt pathway is activated in both angiosarcoma of bone and soft tissue, however, with a different cause; PTEN expression is decreased in angiosarcoma of bone, whereas angiosarcomas of soft tissue show overexpression of KIT. Our findings support that angiosarcomas are a heterogeneous group of vascular malignancies. Both angiosarcoma of bone and soft tissue may benefit from therapeutic strategies targeting the PI3K/Akt pathway. However, interference with TGF-β signaling may be specifically relevant in angiosarcoma of bone.
Similar content being viewed by others
Main
Angiosarcoma is a rare malignant neoplasm composed of cells that demonstrate endothelial differentiation accounting for <1% of all sarcomas.1, 2 Although angiosarcoma can occur at any anatomical site throughout the body, it is most commonly found in the skin of the head and neck region and its underlying superficial soft tissue.1, 2 In all, 20–40% are located within the deep muscles of the extremities.1, 2, 3, 4 Angiosarcoma of bone is extremely rare and accounts for <1% of all malignant bone tumors.5, 6, 7 Angiosarcomas present with widely varying signs and symptoms. Although their clinical behavior is rather unpredictable, it has been generally accepted that they can be extremely aggressive.1, 2, 8, 9, 10, 11
Angiosarcomas constitute a highly heterogeneous group of tumors. At present, there is an increasing role for cellular and molecular features in the classification of tumors.1 These features do not only have an impact on the identification and diagnosis of tumors, but can as well have some therapeutic implications.12 So far, there are relatively few studies investigating cellular and molecular changes within angiosarcoma of bone. Single-case reports and small studies have shown the possible involvement of cell cycle regulators, such as cyclin D1,13 and tumor-suppressor genes such as CDKN2A and TP53,1, 14, 15, 16, 17, 18, 19, 20, 21 in sporadic angiosarcoma of skin, soft tissue and visceral angiosarcomas suggesting a possible role in tumorigenesis in a subset of angiosarcomas. Moreover, a subset of angiosarcomas harbor specific genetic alterations based on either their anatomical site or exposure to radiation.22, 23, 24 In that perspective, high levels of MYC amplification are found in 55–100% of angiosarcomas secondary to radiation exposure or chronic lymphedema after breast surgery and radiation, which is in 25% associated with FLT4 (FMS-like tyrosine kinase-4 encoding for VEGFR3) amplification.22, 23, 24 In addition, KDR (kinase insert domain receptor, VEGFR2) mutations are present in 10% of angiosarcoma of the breast, either primary or secondary to radiation exposure.22, 23 These findings implicate that, based on tumor-specific alterations, angiosarcoma of soft tissue is highly heterogeneous. In angiosarcoma of bone, molecular studies are absent. Therefore, it is still unclear whether angiosarcoma of bone is a true separate entity or is similar to primary angiosarcoma of deep soft tissues. We therefore examined the expression of a large panel of oncogenes, tumor-suppressor genes, and signaling molecules and performed hotspot mutation analysis for PIK3CA in angiosarcoma of bone. Results were compared with angiosarcoma of soft tissue. Our aim was to identify potential pathways involved in the development of angiosarcoma of bone that could serve as potential target for treatment.
Materials and methods
Clinicopathological Data
Formalin-fixed paraffin-embedded tissue of 37 tumors from 33 patients, with the confirmed diagnosis of angiosarcoma of bone were collected from the archives of the Department of Pathology of the Rizzoli Institute, Bologna, Italy (33 tumors from 30 patients), and University Hospitals, Leuven, Belgium (4 tumors from 3 patients) as previously well characterized and described.25 For comparison, 20 angiosarcomas of soft tissue from 20 patients were collected from the archives of the Department of Pathology of University Hospitals, Leuven, Belgium (14 tumors from 14 patients) and Leiden University Medical Center (6 tumors from 6 patients). Fresh frozen tumor tissue of six angiosarcomas of soft tissue (from six patients) was available for mutation analysis. All specimens were handled according to the ethical guidelines described in ‘Code for Proper Secondary Use of Human Tissue in the Netherlands’ of the Dutch Federation of Medical Scientific Societies.
Tissue Microarray
Four tissue microarrays were assembled by standard procedures26, 27 using a 2 mm-diameter punch (3DHistech, Budapest, Hungary; three tissue microarrays) or a 0.6 mm-diameter punch (Beecher Instruments, Silver Spring, MD, USA; one tissue microarray) containing 37 angiosarcomas of bone and 20 angiosarcomas of soft tissue with four cores of each lesion whenever possible. Also six tissue cores of tonsil, seven tissue cores of bowel and three cores of liver were included for orientation and control purposes. Using a tape-transfer system (Instrumedics, Hackensack, NJ, USA), 4-μm sections were transferred to glass slides.
Immunohistochemistry
Immunohistochemistry was performed on tissue microarray slides with different antibodies for oncogenes, tumor-suppressor genes and signaling molecules, which were selected based on their possible role in angiosarcoma of soft tissue, or based on their role in angiogenesis (Table 1). Immunohistochemical reactions were performed according to standard laboratory methods.28 For each antibody, a positive and negative external control was included. The antibodies, their sources, antigen retrieval methods, dilutions, positive and negative external controls used are documented in Table 1. As negative control, sections were stained without adding the primary antibody. The intensity (0=no staining, 1=weak, 2=moderate, 3=strong) and percentage of positive neoplastic cells (0=0%, 1=1–24%, 2=25–49%, 3=50–74%, 4=75–100%) were evaluated. Lost tissue cores were excluded from the analysis. As decalcification could compromise the immunohistochemical result, we attempt to counteract this phenomenon by excluding the tissue samples with a negative internal control. The sum of intensity and percentage was used for analysis. Data were collected through homemade software.29
DNA Isolation and Mutation Detection
DNA was isolated from formalin-fixed paraffin-embedded material from 13 angiosarcomas of bone, and from fresh frozen material from six angiosarcomas of soft tissue, as described previously.30 The Custom TaqMan Assay Design Tool (Applied Biosystems, Nieuwerkerk a/d Ijssel, The Netherlands) was used to detect seven different KRAS, three PIK3CA and one BRAF mutations as described previously.31 Raw data from the LC480 software were imported into an inhouse-created Microsoft Excel 2003 spreadsheet to define the mutation status as described in detail previously.31
Statistical Analysis
The results of the immunohistochemical analysis were correlated to clinicopathological data available for the angiosarcomas of bone, which were documented previously.25 The correlation between immunohistochemical markers and the specific location of the tumors (bone versus soft tissue) was performed using global testing and the non-parametric Mann–Whitney U two-independent sample test. Global testing was used to determine whether a pre-specified group of genes were differentially expressed between angiosarcoma of bone and soft tissue.32 Kruskal–Wallis H test (non-parametric K independent sample test) was performed to evaluate the correlation between immunohistochemical markers within the different subgroups based on the origin (bone, soft tissue, head and neck region, or visceral involvement). Subsequently, immunohistochemical markers were combined into different pathways and by global testing we evaluated the correlation between these pathways and the two different groups of angiosarcoma (bone versus soft tissue) and corrected the results for multiple testing. Values of P<0.005 (corrected for multiple testing) were considered statistically significant. The data were analyzed using R (R Development Core Team (2011). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/), Global testing32 and SPSS 17.0 software (SPSS, Chicago, IL, USA). As described previously, follow-up data were available of 31 patients with angiosarcoma of bone.25 Survival was evaluated by Kaplan–Meier analysis and log-rank test. Values of P≤0.05 were considered statistically significant.
Results
Patient Characteristics
Patient characteristics of both angiosarcoma of bone and soft tissue are summarized in Table 2. All patient characteristics for angiosarcoma of bone have been described in detail previously.25 Based on the location of the angiosarcoma of soft tissue, the patients could be divided into three different subgroups: seven patients had an angiosarcoma located within the head and neck region, eight patients had an angiosarcoma of deep soft tissue and five patients had a visceral angiosarcoma. None of the patients had a history of radiation, that is, for other cancers, and/or chronic lymphedema at the location of the angiosarcoma.
Cell Cycle Regulators in Angiosarcoma of Bone
Nearly 55% of the angiosarcoma of bone showed a defect in the retinoblastoma (Rb) pathway, affecting either CDKN2A, cyclin D1 or both. A total loss of protein expression of CDKN2A (sumscore 0, with positive internal control) was detected in 49% (16/33) of angiosarcomas of bone. Six percent (2/33) of the angiosarcomas of bone demonstrated overexpression of cyclin D1 (sumscore≥6), of which one tumor showed normal CDKN2A expression and one tumor showed a total loss of CDKN2A protein expression. Kaplan–Meier survival analysis demonstrates that patients showing loss of CDKN2A expression (sumscore 0) have a significantly worse survival (P=0.017; Figure 1). Overexpression of CDK4 (sumscore≥6) did not occur in angiosarcoma of bone (0%). None of the angiosarcomas of bone showed overexpression (sumscore≥6) of TP53 (Figure 2) or MDM2. The anti-apoptotic protein BCL-2 was only weakly expressed in the majority of angiosarcoma of bone.
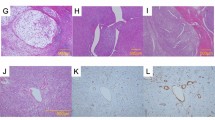
Dot plots and immunohistochemical results for TP53, CD117 en PTEN in angiosarcoma of bone (ASB) compared with its soft tissue (ASST) counterpart. (a) TP53 shows no overexpression in angiosarcoma of bone, whereas three angiosarcomas of soft tissue show clear TP53 overexpression (sumscore>6). Although some angiosarcomas of bone show positivity for TP53, clear overexpression was not seen (b), in contrast to angiosarcoma of soft tissue (c). (d) Angiosarcomas of bone show a significant lower expression of CD117 than angiosarcomas of soft tissue; (e) weak expression of CD117 in angiosarcoma of bone compared with high expression in angiosarcoma of soft tissue (f). (g) A subset of angiosarcomas of bone show a decrease in expression (sumscore≤3) of PTEN. Low expression (h) and normal expression (i) of PTEN in angiosarcoma of bone, as well as low expression of PTEN in an angiosarcoma of soft tissue (j) (scale bars 50 μm).
Signaling Pathways in Angiosarcoma of Bone
Transforming growth factor-β (TGF-β) signaling was highly active in angiosarcoma of bone; all tumors showed immunoreactivity for the ligand TGF-β1 and the downstream targets phospho-Smad2 and plasminogen activator inhibitor type 1 (PAI-1; Figure 3). Dot plots for all antibodies are shown in Figure 4. In addition, nuclear expression of phospho-Smad1, which is indicative of active BMP signaling, was found in 68% of the angiosarcomas of bone (sumscore>0). Endoglin (CD105) was expressed (sumscore>0) by the tumor cells in the majority (97%) of the tumors. Moreover, 41% of the angiosarcomas of bone showed a decrease in expression of PTEN (sumscore≤3) suggesting involvement of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway (Figure 2). There was no evidence of active canonical Wnt signaling as we did not find nuclear localization of β-catenin in the vast majority of the tumors. As for cell survival signaling, both the hypoxia-inducible factor-1α as well as the glucose transporter-1 were expressed in only a few tumors. On the other hand, VEGF was widely and strongly expressed (Figure 4).
Difference in protein expression (sumscore) for TGF-β (a), phosphoSmad2 (b), PAI-1 (c) and phosphoSmad1 (d) between angiosarcoma of bone (ASB) and soft tissue (ASST) illustrated by dot plots. (e) Angiosarcoma of bone showing a strong TGF-β expression. (f) Angiosarcoma of bone showing a strong phosphoSmad2 expression (scale bars: 50 μm).
Angiosarcoma of Bone versus Angiosarcoma of Soft Tissue
When combining immunohistochemical markers belonging to the same pathway essential in cell signaling, proliferation and survival, global testing demonstrates a significant difference in expression for TGF-β pathway and PI3K/Akt pathway between angiosarcoma of bone and soft tissue (Table 3). The TGF-β signaling is more active in angiosarcoma of bone compared with its soft tissue counterpart as shown by global testing, which is based on difference in phospho-Smad2 expression between angiosarcoma of bone and soft tissue (Figure 5). The PI3K/Akt pathway seems to be involved in both angiosarcoma of bone and soft tissue, but through a different mechanism. The PI3K/Akt pathway in angiosarcoma of bone is affected by decreased expression of PTEN (sumscore≤3), whereas in angiosarcoma of soft tissue this is mainly associated with overexpression of CD117. Decreased expression of PTEN is seen in 11 of 27 angiosarcomas of bone (41%) as compared with 1 of 15 angiosarcomas of soft tissue (7%; P=0.049; Figure 2). Only 17% (5/29) of the angiosarcomas of bone show expression of CD117, compared with 90% (17/19) of the angiosarcomas of soft tissue (Figure 2). Global testing indeed demonstrates a significant difference in the overall immunohistochemical expression pattern (including all markers tested) between angiosarcoma of bone and soft tissue (P=0.0004), which is mainly because of CD117 being predominantly expressed in soft tissue angiosarcoma (P=0.00001; Figure 5). In addition, global testing shows that there is an apparent difference in expression for phospho-Smad2, PTEN and TGF-β between both tumor groups (Figure 5). The difference in protein expression between angiosarcoma of bone and soft tissue of all antibodies are illustrated by dot plots documented in Figure 4. In 75% of the angiosarcomas of soft tissue, the Rb pathway is affected, either by loss of CDKN2A (sumscore 0, 12/20; 60%) and/or overexpression of cyclin D1 (sumscore≥6, 5/19; 26%). Only six percent (3/18) of the angiosarcomas of soft tissue show overexpression of TP53 (sumscore≥6). Tumors overexpressing TP53 were mainly located in the head and neck region (2/3).
Mutation Analysis in Angiosarcoma
As the PI3K/Akt signaling pathway seems to be differently activated between angiosarcoma of bone and soft tissue, we continued to analyze for mutations in PIK3CA. However, no hotspot mutations were detected in angiosarcoma of bone or soft tissue. Moreover, there were no mutations detected for KRAS and BRAF.
Discussion
Dysregulation of the Rb/CDKN2A and/or TP53 pathway are the most common alterations found in almost all types of human cancer.33 Our results show that in angiosarcoma of bone, the Rb pathway is disrupted in 55% of the cases, mainly by loss of protein expression of CDKN2A (49%) or overexpression of cyclin D1 (6%), contributing to uncontrolled cell proliferation and tumorigenesis. We additionally show that, unlike in angiosarcoma of soft tissue, in angiosarcoma of bone the TP53 pathway is not important. The involvement of the Rb pathway in a small majority of angiosarcoma of bone is consistent with the hypothesis that angiosarcomas belong to the category of sarcomas with complex genomic profiles, which frequently have alterations in the Rb/CDKN2A and/or TP53 pathways.34, 35
Moreover, inactivation of CDKN2A is associated with a more aggressive behavior in many cancers, including for instance Ewing sarcoma34, 36, 37 and osteosarcoma.38, 39 Here, we demonstrate that also patients with angiosarcoma of bone showing loss of expression of CDKN2A have a significantly worse prognosis. To date, few studies have reported the possible role of CDKN2A and TP53 in tumor development in a subset of angiosarcomas of soft tissue.15, 16, 17, 18, 19, 20, 37, 40, 41, 42 Recently, it has been shown that Ink4/Arf deficiency in mice is associated with the development of angiosarcoma, lymphoma and fibrosarcoma.43
By global testing, we demonstrate that the TGF-β pathway (TGF-β1/phospho-Smad2/PAI-1) is highly active in angiosarcoma of bone and therefore could have an important role in tumorigenesis. TGF-β1 is the major isoform present in bone and, among others, involved in bone formation.44 It binds and activates ALK5, one of its specific type I receptors, and thereby inducing phosphorylation of Smad2/Smad3, resulting in the expression of the PAI-1.45 To date, it is accepted that the TGF-β signaling pathway has an important role during embryogenesis, cell differentiation and maintenance of homeostasis during adult life, but it also prohibits uncontrolled cell proliferation.45, 46 Deregulation of this pathway causes resistance to the TGF-β-mediated growth arrest and gives rise to malignancies.47, 48, 49 However, the molecular mechanisms provoking this deregulation, that is, genetic and epigenetic aberrations, and the underlying oncogenic activities of TGF-β still remain unclear.49 Our findings suggest possible therapeutic options for angiosarcoma of bone, because inhibitors of the TGF-β pathway (Figure 6) are available.50 By now, multiple pre-clinical and clinical studies are investigating the inhibition of the oncogenic TGF-β signaling as a therapeutic approach in different types of cancer.50
A simplified overview of the TGF-β pathway (left) and the PI3K/Akt (right) pathway and possible therapeutic strategies to inhibit the pathway. TGF-β can be inhibited by ligand traps (soluble receptor ectodomain constructs), neutralizing antibodies and ALK5/TβRII kinase inhibitors. In addition, ligand production can be prevented by blocking translation of TGF-β mRNA with antisense oligonucleotides. The PI3K/Akt pathway can be inhibited by pure PI3K inhibitors, dual PI3K/mTOR inhibitors, AKT inhibitors, and mTOR inhibitors, for example, rapamycin and analogs (rapalogs). More specifically, receptor tyrosine kinases such as KIT can be blocked by a tyrosine kinase inhibitor (TKI).
Pathway analysis further demonstrates that the PI3K/Akt pathway, is highly active in both angiosarcoma of bone and soft tissue (Figure 6). However, the differences in protein expression suggest that activation occurs through different mechanisms. In angiosarcoma of bone, 41% show decreased expression of the tumor-suppressor PTEN, compared with only 7% in angiosarcoma of soft tissue. However, in angiosarcoma of soft tissue, the tyrosine kinase receptor KIT is overexpressed in 90%, whereas this is infrequent (17%) in angiosarcoma of bone. This is corresponding to previous studies, reporting that 20 to 58% of the soft tissue angiosarcomas show CD117 expression,51, 52 whereas normal adult endothelial cells lack CD117 overexpression.52, 53, 54 To date, there are no KIT and/or PDGFRA mutations detected in angiosarcoma of soft tissue.52, 54, 55 Of note, there is one report of a very good response to imatinib (Glivec, a specific tyrosine kinase inhibitor) in angiosarcoma of soft tissue.55 We show limited expression of CD117 in a minority of angiosarcoma of bone, suggesting that a specific tyrosine kinase-inhibitor, such as imatinib, could be a therapeutic option for angiosarcoma of soft tissue but not for angiosarcoma of bone.
In angiosarcoma of bone on the other hand, we demonstrate decreased expression of PTEN. To date, there is only one report of a human PTEN gene mutation in one out of two angiosarcomas of the liver containing a single-nucleotide substitution in exon 7.56 A recent publication demonstrates that about 10% of the PTEN-haploinsufficient zebrafishes develop hemangiosarcomas and these tumors have an activated PI3K/Akt signaling,57 thereby illustrating the possible role of PTEN in the development of vascular tumors. Although it would be interesting to evaluate possible PTEN mutations within angiosarcoma of bone, mutation analysis could not be performed in our study, because there was not enough more DNA available.
The PI3K/Akt pathway is a signal transduction cascade that has a role and regulates a large variety of important physiological processes, such as cell proliferation and metabolism, adhesion, survival, protein synthesis, motility and angiogenesis.58 Genetic aberrations, such as PIK3CA mutation, have been described, which constitutively activate this pathway and thereby potentially induce malignancies.58, 59 As the PI3K/Akt pathway is activated in both angiosarcoma of bone and soft tissue, we additionally examined PIK3CA for hotspot mutations. No mutations were found. Of note, recent publications have shown the presence of PIK3CA mutations in vascular anomalies and benign vascular lesions.60, 61
The activated PI3K/Akt pathway in angiosarcomas provides a rationale for the use of inhibitors of the PI3K/Akt pathway in these patients (Figure 6).58, 62 The serine/threonine kinase mammalian target of rapamycin (mTOR) is a kinase downstream in the PI3K/Akt pathway and therefore an important regulator of cell proliferation, metabolism and protein synthesis. The mTOR activity can be inhibited by rapamycin (sirolimus) and analogs (rapalogs), for example, Temsirolimus/CCI-779 and everolimus/RAD001. Beuvinck et al demonstrated in human tumor xenografts that everolimus inhibits angiogenesis by preventing the proliferation of endothelial cells.63 So far, there is only one report demonstrating the anti-proliferative effect of rapamycin in angiosarcoma cell lines.64 In addition, new drugs such as pure PI3K inhibitors, dual PI3K-mTOR inhibitors, Akt inhibitors and mTOR kinase inhibitors are evolving and passing through the early phases of clinical development.58 Our findings would support further studies into the effectiveness of these inhibitors in angiosarcoma of bone. However, thus far, no cell lines derived from angiosarcoma of bone are available.
In conclusion, we demonstrate that angiosarcoma of bone is different from angiosarcoma of soft tissue at the molecular level, suggesting that the underlying mechanism of tumorigenesis between both groups may be different. This supports the previous finding that angiosarcomas are a heterogeneous group of malignant vascular tumors with possible different subgroups and corresponding expression profiles.22, 23 We demonstrate that in 55% of the angiosarcoma of bone the Rb pathway is involved in tumorigenesis, mainly by the loss of protein expression of CDKN2A, which seems to be related with a significantly worse prognosis. The TP53 pathway seems of no importance in angiosarcoma of bone. Moreover, by global testing we demonstrate that the TGF-β pathway is more active in angiosarcoma compared with its soft tissue counterpart. The PI3K/Akt pathway is active in both angiosarcoma of bone and soft tissue, and we here demonstrate that while in angiosarcoma of soft tissue overexpression of KIT is involved, in angiosarcoma of bone PTEN expression is decreased. Our results provide rationale for therapeutic strategies including TGF-β and/or PI3K/mTOR inhibitors to improve the generally poor prognosis of patients with angiosarcoma of bone.
References
Fletcher CDM, Unni KK, Mertens F . World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, France, 2002.
Weiss SW, Goldblum JR . Malignant vascular tumors In Weiss SW, Goldblum JR (eds). Enzinger & Weiss's Soft Tissue Tumors. Mosby, an affiliate of Elsevier: Philadelphia, 2008;703–732.
Abraham JA, Hornicek FJ, Kaufman AM et al. Treatment and outcome of 82 patients with angiosarcoma. Ann. Surg. Oncol. 2007;14:1953–1967.
Meis-Kindblom JM, Kindblom LG . Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol 1998;22:683–697.
Dorfman HD, Czerniak B . Vascular lesions In Dorfman HD, Czerniak B eds. Bone Tumors 1st edn. Mosby: St Louis, MO, USA, 1998;729–814.
Huvos AG . Angiosarcoma of bone (epithelioid hemangioendothelioma; malignant hemangioendothelioma) In Huvos AG (ed). Bone Tumors. Diagnosis, treatment, and prognosis second edition ed. W.B. Saunders: Philadelphia, 1991;579–598.
Mulder JD, Schütte HE, Kroon HM et al Hemangioendothelioma and Hemangioendotheliosarcoma. Radiologic Atlas of Bone Tumors 1st edn. Elsevier: Amsterdam, 1993;249–254.
Deshpande V, Rosenberg AE, O'Connell JX et al. Epithelioid angiosarcoma of the bone: a series of 10 cases. Am J Surg Pathol 2003;27:709–716.
Evans HL, Raymond AK, Ayala AG . Vascular tumors of bone: a study of 17 cases other than ordinary hemangioma, with an evaluation of the relationship of hemangioendothelioma of bone to epithelioid hemangioma, epithelioid hemangioendothelioma, and high-grade angiosarcoma. Hum Pathol 2003;34:680–689.
O'Connell JX, Nielsen GP, Rosenberg AE . Epithelioid vascular tumors of bone: a review and proposal of a classification scheme. Adv Anat Pathol 2001;8:74–82.
Wenger DE, Wold LE . Malignant vascular lesions of bone: radiologic and pathologic features. Skeletal Radiol 2000;29:619–631.
Bovée JVMG, Hogendoorn PCW . Molecular pathology of sarcomas: concepts and clinical implications. Virchows Arch 2010;456:193–199.
Lin N, Uchi H, Moroi Y et al. Significance of the expression of phosphorylated signal transducer and activator of transcription-3, -Akt, and -cyclin D1 in angiosarcoma. J Dermatol Sci 2007;48:64–66.
Domfeh AB, Fichera M, Hunt JL . Allelic loss of 3 different tumor suppressor gene loci in benign and malignant endothelial tumors of the head and neck. Arch Pathol Lab Med 2006;130:1184–1187.
Zu Y, Perle MA, Yan Z et al. Chromosomal abnormalities and p53 gene mutation in a cardiac angiosarcoma. Appl Immunohistochem Mol Morphol 2001;9:24–28.
Amo Y, Masuzawa M, Hamada Y et al. Serum levels of vascular endothelial growth factor in a haemangiosarcoma patient with a newly typed p53 gene point mutation. Br J Dermatol 2000;143:1118–1119.
Garcia JM, Gonzalez R, Silva JM et al. Mutational status of K-ras and TP53 genes in primary sarcomas of the heart. Br J Cancer 2000;82:1183–1185.
Zietz C, Rossle M, Haas C et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Pathol 1998;153:1425–1433.
Naka N, Tomita Y, Nakanishi H et al. Mutations of p53 tumor-suppressor gene in angiosarcoma. Int J Cancer 1997;71:952–955.
Soini Y, Welsh JA, Ishak KG et al. p53 mutations in primary hepatic angiosarcomas not associated with vinyl chloride exposure. Carcinogenesis 1995;16:2879–2881.
Przygodzki RM, Finkelstein SD, Keohavong P et al. Sporadic and thorotrast-induced angiosarcomas of the liver manifest frequent and multiple point mutations in K-ras-2. Lab Invest 1997;76:153–159.
Antonescu CR, Yoshida A, Guo T et al. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res 2009;69:7175–7179.
Guo T, Zhang L, Chang NE et al. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes. Cancer 2011;50:25–33.
Manner J, Radlwimmer B, Hohenberger P et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol 2010;176:34–39.
Verbeke SLJ, Bertoni F, Bacchini P et al. Distinct histological features characterize primary angiosarcoma of bone. Histopathology 2011;58:254–264.
Kononen J, Bubendorf L, Kallioniemi A et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998;4:844–847.
Goethals L, Perneel C, Debucquoy A et al. A new approach to the validation of tissue microarrays. J Pathol 2006;208:607–614.
Pansuriya TC, Oosting J, Krenacs T et al. Genome-wide analysis of Ollier disease: is it all in the genes? Orphanet J Rare Dis 2011;6:2.
Romeo S, Debiec-Rychter M, Van Glabbeke M et al. Cell cycle/apoptosis molecule expression correlates with imatinib response in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res 2009;15:4191–4198.
de Jong D, Verbeke SLJ, Meijer D et al. Opening the archives for state of the art tumour genetic research: sample processing for array-CGH using decalcified, formalin-fixed, paraffin-embedded tissue-derived DNA samples. BMC Res Notes 2011;4:1.
Van Eijk R, Licht J, Schrumpf M et al. Rapid KRAS, EGFR, BRAF and PIK3CA mutation analysis of fine needle aspirates from non-small-cell lung cancer using allele-specific qPCR. PLoS One 2011;6:e17791.
Goeman JJ, van de Geer SA, de Kort F et al. A global test for groups of genes: testing association with a clinical outcome. Bioinformatics 2004;20:93–99.
Sherr CJ, McCormick F . The RB and p53 pathways in cancer. Cancer Cell 2002;2:103–112.
Helman LJ, Meltzer P . Mechanisms of sarcoma development. Nat Rev Cancer 2003;3:685–694.
Perot G, Chibon F, Montero A et al. Constant p53 pathway inactivation in a large series of soft tissue sarcomas with complex genetics. Am J Pathol 2010;177:2080–2090.
Honoki K, Stojanovski E, McEvoy M et al. Prognostic significance of p16 INK4a alteration for Ewing sarcoma: a meta-analysis. Cancer 2007;110:1351–1360.
Orlow I, Drobnjak M, Zhang ZF et al. Alterations of INK4A and INK4B genes in adult soft tissue sarcomas: effect on survival. J Natl Cancer Inst 1999;91:73–79.
Mohseny AB, Szuhai K, Romeo S et al. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J Pathol 2009;219:294–305.
Mohseny AB, Tieken C, van der Velden PA et al. Small deletions but not methylation underlie CDKN2A/p16 loss of expression in conventional osteosarcoma. Genes Chromosomes. Cancer 2010;49:1095–1103.
Weihrauch M, Markwarth A, Lehnert G et al. Abnormalities of the ARF-p53 pathway in primary angiosarcomas of the liver. Hum Pathol 2002;33:884–892.
Tannapfel A, Weihrauch M, Benicke M et al. p16INK4A-alterations in primary angiosarcoma of the liver. J Hepatol 2001;35:62–67.
Zindy F, Nilsson LM, Nguyen L et al. Hemangiosarcomas, medulloblastomas, and other tumors in Ink4c/p53-null mice. Cancer Res 2003;63:5420–5427.
Yang J, Kantrow S, Sai J et al. Ikk4a/Arf inactivation with activation of the NF-kappaB/IL-6 pathway is sufficient to drive the development and growth of angiosarcoma. Cancer Res 2012;72:4682–4695.
Janssens K, ten Dijke P, Janssens S et al. Transforming growth factor-beta1 to the bone. Endocr Rev 2005;26:743–774.
Lebrin F, Deckers M, Bertolino P et al. TGF-beta receptor function in the endothelium. Cardiovasc Res 2005;65:599–608.
Goumans MJ, Mummery C . Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 2000;44:253–265.
Galliher AJ, Neil JR, Schiemann WP . Role of transforming growth factor-beta in cancer progression. Future Oncol 2006;2:743–763.
Blobe GC, Schiemann WP, Lodish HF . Role of transforming growth factor beta in human disease. N Engl J Med 2000;342:1350–1358.
Tian M, Neil JR, Schiemann WP . Transforming growth factor-beta and the hallmarks of cancer. Cell Signal 2011;23:951–962.
Hawinkels LJ, Ten Dijke P . Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors 2011;29:140–152.
Hornick JL, Fletcher CD . Immunohistochemical staining for KIT (CD117) in soft tissue sarcomas is very limited in distribution. Am J Clin Pathol 2002;117:188–193.
Miettinen M, Sarlomo-Rikala M, Lasota J . KIT expression in angiosarcomas and fetal endothelial cells: lack of mutations of exon 11 and exon 17 of C-kit. Mod Pathol 2000;13:536–541.
Lammie A, Drobnjak M, Gerald W et al. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem 1994;42:1417–1425.
Miettinen M, Lasota J . KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol 2005;13:205–220.
Kiesel H, Muller AM, Schmitt-Graeff A et al. Dramatic and durable efficacy of imatinib in an advanced angiosarcoma without detectable KIT and PDGFRA mutations. Cancer Biol Ther 2009;8:319–321.
Tate G, Suzuki T, Mitsuya T . Mutation of the PTEN gene in a human hepatic angiosarcoma. Cancer Genet Cytogenet 2007;178:160–162.
Choorapoikayil S, Kuiper RV, de Bruin A et al. Haploinsufficiency of the genes encoding the tumor suppressor Pten predisposes zebrafish to hemangiosarcoma. Dis Model Mech 2012;5:241–247.
Markman B, Dienstmann R, Tabernero J . Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs. Oncotarget 2010;1:530–543.
Ligresti G, Militello L, Steelman LS et al. PIK3CA mutations in human solid tumors: role in sensitivity to various therapeutic approaches. Cell Cycle 2009;8:1352–1358.
Kurek KC, van Ruler MAJH, van den Akker B et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors. Am J Pathol advance online publication, 26 February 2013 (e-pub ahead of print).
Kurek KC, Luks VL, Ayturk UM et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am J Hum Genet 2012;90:1108–1115.
Carew JS, Kelly KR, Nawrocki ST . Mechanisms of mTOR inhibitor resistance in cancer therapy. Target Oncol 2011;6:17–27.
Campone M, Levy V, Bourbouloux E et al. Safety and pharmacokinetics of paclitaxel and the oral mTOR inhibitor everolimus in advanced solid tumours. Br J Cancer 2009;100:315–321.
Bundscherer A, Vogt T, Kohl G et al. Antiproliferative effects of rapamycin and celecoxib in angiosarcoma cell lines. Anticancer Res 2010;30:4017–4023.
Acknowledgements
The continuous support of the Netherlands Committee on Bone Tumors is highly acknowledged. We thank Drs EL Staals and P Picci for their contribution of patient data, Inge Briaire—de Bruijn for the expert technical assistance, Ronald van Eijk and Tom van Wezel for help with mutation analysis and Professor Dr Pancras CW Hogendoorn and Professor Dr Peter ten Dijke for valuable discussions. The studies were financially supported by the Dutch Cancer Society (Grant number: UL 2007–3975 to SLJV) and EuroBoNet consortium (contract number LSHC-CT-2006-018814), a European Commission granted Network of Excellence for studying the pathology and genetics of bone tumors, to the departments of pathology of the Rizzoli Institute Bologna, University Hospitals Leuven, Semmelweis University Budapest and Leiden University Medical Center Leiden.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Verbeke, S., Bertoni, F., Bacchini, P. et al. Active TGF-β signaling and decreased expression of PTEN separates angiosarcoma of bone from its soft tissue counterpart. Mod Pathol 26, 1211–1221 (2013). https://doi.org/10.1038/modpathol.2013.56
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2013.56
Keywords
This article is cited by
-
Endothelial cell malignancies: new insights from the laboratory and clinic
npj Precision Oncology (2017)