Abstract
Solitary fibrous tumor (SFT) is composed of spindled to ovoid cells in a patternless architecture with prominent stromal collagen and hemangiopericytoma-like vessels. Some tumors show hypercellularity, nuclear atypia, and significant mitotic activity; the latter feature in particular often portends an aggressive clinical course. SFT can sometimes be difficult to distinguish from other benign mesenchymal tumors and sarcomas. The most characteristic (albeit nonspecific) immunohistochemical finding in SFT is CD34 expression. A NAB2–STAT6 gene fusion, resulting in a chimeric protein in which a repressor domain of NGFI-A binding protein 2 (EGR1 binding protein 2) (NAB2) is replaced with a carboxy-terminal transactivation domain from signal transducer and activator of transcription 6, interleukin-4 induced (STAT6), was recently identified as a consistent finding in SFT. However, as these genes are located in close proximity on 12q13, this fusion can only rarely be detected by conventional chromosomal banding or fluorescence in situ hybridization analysis. Nuclear expression of the carboxy terminal part of STAT6 is a consistent finding in SFT of the meninges (so-called ‘meningeal hemangiopericytoma’). We investigated STAT6 expression by immunohistochemistry in SFTs and other soft tissue tumors arising outside the central nervous system to validate the diagnostic utility of this novel marker. Whole-tissue sections of 231 tumors were evaluated, including 60 cases of SFT as well as other benign and malignant mesenchymal neoplasms and sarcomatoid mesotheliomas. Fifty-nine of 60 SFT cases (98%) showed nuclear expression of STAT6, which was usually diffuse and intense. All other tumor types were negative for STAT6, except for three dedifferentiated liposarcomas and one deep fibrous histiocytoma, which showed weak staining. In conclusion, STAT6 is a highly sensitive and almost perfectly specific immunohistochemical marker for SFT and can be helpful to distinguish this tumor type from histologic mimics.
Similar content being viewed by others
Main
Solitary fibrous tumor (SFT) is an anatomically ubiquitous fibroblastic neoplasm that most often affects middle-aged adults. SFT was originally described in 1931 as a pleural tumor termed ‘hemangiopericytoma’,1 but over time it has been increasingly recognized that SFT may arise at a wide range of anatomical locations. Of extrapleural tumors, ∼40% arise in subcutaneous tissue, with the remaining cases occurring in deep soft tissues, retroperitoneum, mediastinum, abdominal cavity, and meninges among other sites.
In its classic form, SFT shows variably hypocellular and hypercellular areas composed of spindled to ovoid tumor cells with scant cytoplasm and indistinct cell borders, in a ‘patternless’ architecture. Prominent branching hemangiopericytoma-like vasculature is a characteristic finding, and the stroma is usually collagenous and hyalinized, occasionally with myxoid areas.2, 3, 4, 5 Lipomatous (fat-forming) differentiation may also occur. The tumor cells of SFT are usually bland, lacking significant atypia or pleomorphism, but atypical, cellular, and malignant forms of SFT exist, reflecting the variable (although sometimes unpredictable) biological potential of this tumor.
SFT can sometimes be difficult to distinguish from some other benign mesenchymal tumors and spindle cell sarcomas, particularly in small biopsy samples. Immunohistochemistry using existing markers is of variable utility in the differential diagnosis. Traditionally, CD34 expression is the most consistent reported finding in SFT, present in up to 95% of cases. However, CD34 expression is also common in other tumors that may mimic SFT, such as soft tissue perineurioma, dermatofibrosarcoma protuberans, and spindle cell lipoma. Furthermore, SFT shows nuclear expression of β-catenin in 40% of cases, which may lead to confusion with desmoid fibromatosis.6 SFT also shows reactivity for EMA in 20–30% of cases7 and cytoplasmic staining for CD99 in ∼70% of cases.7, 8 Positivity for BCL2 is seen in virtually all SFTs,7, 9, 10 but is a nonspecific finding, as expression is also observed in a variety of other mesenchymal neoplasms.9
Until recently, very little was known about the molecular genetics of SFT. However, using whole-exome and transcriptome sequencing, three groups have recently identified NAB2–STAT6 gene fusions in the vast majority of SFTs.11, 12, 13 These genes are located close together on chromosome 12 and are transcribed in opposite directions. The fusion product results from an inversion at the 12q13 locus, which fuses NAB2 and STAT6. The resultant fusion protein, in which a repressor domain of NGFI-A binding protein 2 (EGR1 binding protein 2) (NAB2) is replaced by a transactivation domain from the carboxy terminal part of signal transducer and activator of transcription 6, interleukin-4 induced (STAT6), is believed to act as a transcriptional activator through early growth response (EGR)-mediated pathways. In a study of mesenchymal tumors of the central nervous system, Schweizer et al15 have identified the same fusion gene in meningeal SFTs and hemangiopericytomas, confirming that meningeal ‘hemangiopericytoma’ is indeed a hypercellular variant of SFT (similar to the situation at other anatomical sites);14 the authors also demonstrated that nuclear STAT6 protein overexpression by immunohistochemistry is restricted to this tumor type among meningeal tumors.
Although in most cases the diagnosis of SFT is relatively straightforward, some tumors may mimic other benign mesenchymal tumors or sarcomas, and a subset of SFTs lack expression of CD34. In this study, we investigated STAT6 protein expression by immunohistochemistry in SFT and histologic mimics to validate the utility of STAT6 as a diagnostic marker.
Materials and methods
Cases were retrieved from the surgical pathology and consult files of Brigham and Women’s Hospital, Boston, MA and Skåne University Hospital, Lund, Sweden, and the consult files of one of the authors (CDMF). Representative hematoxylin and eosin-stained slides were reviewed. Cases of SFT were classified according to the WHO Classification of Tumors as conventional (classic), malignant (>4 mitoses per 10 high-power fields, with or without hypercellularity, atypia, and infiltrative growth), and cellular or atypical in the presence of marked diffuse hypercellularity or nuclear atypia, respectively, but without increased mitotic activity.16 In total, whole-tissue sections of 231 tumors were evaluated for expression of STAT6: 60 cases of SFT (including 31 conventional (4 with prominent myxoid stroma), 9 cellular, 5 atypical, and 15 malignant variants), 20 malignant peripheral nerve sheath tumors, 10 sarcomatoid mesotheliomas, 10 cases of desmoid fibromatosis, 10 cellular schwannomas, 20 monophasic synovial sarcomas, 20 cases of dermatofibrosarcoma protuberans, 10 low-grade fibromyxoid sarcomas, 10 soft tissue perineuriomas, 10 deep fibrous histiocytomas, 10 gastrointestinal stromal tumors (spindle cell type), 21 dedifferentiated liposarcomas, 10 spindle cell lipomas, and 10 cellular angiofibromas. Six of the cases of SFT were previously shown to harbor the NAB2–STAT6 fusion gene.13
Immunohistochemistry for STAT6 was performed on 4-μm-thick formalin-fixed paraffin-embedded tissue sections following pressure cooker antigen retrieval (0.01 M citrate buffer; pH, 6.0) using a rabbit polyclonal antibody directed against the C terminus of STAT6 (1:1000; sc-621; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Appropriate positive (SFT with genetically confirmed NAB2–STAT6 gene fusion) and negative controls were used throughout. It is noteworthy that during antibody optimization with low dilutions, weak nuclear staining was observed in some normal cell types (vascular endothelium, lymphocytes), whereas at higher dilutions normal tissues were completely negative, but strong staining was seen in the nuclei of a SFT confirmed to have the NAB2–STAT6 fusion gene. The extent of immunoreactivity was graded according to the percentage of positive tumor cells (0, no staining; 1+, <5%; 2+, 5–25%; 3+, 26–50%; 4+, 51–75%; and 5+, 76–100%), and the intensity of staining was graded as weak, moderate, or strong.
Results
The SFTs arose over a wide anatomic distribution, including 10 in the extremities (6 lower and 4 upper), 10 in the head and neck, 9 in the thoracic cavity (5 pleura, 3 lung, and 1 mediastinum), 7 in the abdominal cavity (including 1 each in the liver, uterus, pancreas, and urinary bladder), 7 in the pelvis, 6 in the retroperitoneum, 6 in the trunk, 4 in the inguinal region, and 1 in the meninges. The results of immunohistochemistry for STAT6 are summarized in Table 1. Fifty-nine of 60 cases of SFT (98%) showed nuclear staining for STAT6, which was usually diffuse (5+ in 41 cases; 4+ in 6 cases; 3+ in 5 cases; 2+ in 3 cases; 1+ in 4 cases) and intense (strong in 40 cases; moderate in 15 cases; and weak in 4 cases; Figure 1). In cases with less than diffuse staining (ie, 1+ or 2+) and in four cases with strong expression, heterogeneous staining in a zonal distribution pattern was often seen, with areas in the center of the tissue sections showing no (or weaker) staining, suggesting that this may be related to incomplete tissue fixation. The one SFT that was negative for STAT6 by immunohistochemistry showed histological features of malignancy, was positive for CD34, and was previously demonstrated to have a NAB2–STAT6 fusion gene. The remaining five SFTs known to harbor the NAB2–STAT6 fusion gene showed diffuse moderate-to-strong expression of STAT6. Of the 59 SFTs that were positive for STAT6, 4 were negative for CD34, 54 were positive, and in 1 case CD34 expression status was unknown.
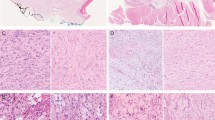
Classic solitary fibrous tumor (SFT) composed of bland spindle cells with dense stromal collagen and branching ‘hemangiopericytoma-like’ vessels (a). Most of the tumor cells show nuclear expression of STAT6, whereas vascular endothelial cells are negative (b). Cellular SFT; the tumor cells are ovoid and there is minimal stromal collagen (c). Diffuse, strong nuclear expression of STAT6 is observed in tumor cells (d). This example of malignant SFT, which showed conspicuous mitotic activity, resembled synovial sarcoma, with monomorphic spindle cells arranged in a somewhat fascicular architecture (e). The tumor cells show nuclear expression of STAT6 (f).
All other tumor types were negative for STAT6 (Figure 2), with the exception of 3 of 21 (14%) dedifferentiated liposarcomas, which showed predominantly weak staining (4+ weak in 1 case; 3+ weak in 1 case; 1+ weak in the well-differentiated component only of 1 case; Figure 3), and 1 of 10 deep fibrous histiocytomas, which showed 1+ weak nuclear staining.
Eight tumors other than SFT (one low-grade fibromyxoid sarcoma, one spindle cell lipoma, one cellular angiofibroma, one cellular schwannoma, two cases of desmoid fibromatosis, and two malignant peripheral nerve sheath tumors) showed a barely perceptible ‘blush’ of staining, but lacked a distinct nuclear pattern of expression and were therefore interpreted as negative.
Discussion
SFT is a fibroblastic neoplasm with variable clinical behavior. Although most SFTs pursue a benign clinical course, recurrence or metastasis develops in 5–10% of patients. The majority of SFTs that behave aggressively show histological features of malignancy, such as >4 mitoses per 10 HPF and hypercellularity, but benign-appearing SFTs can also rarely give rise to metastases.17 Histologically, SFT may resemble some benign soft tissue tumors (eg, soft tissue perineurioma, desmoid fibromatosis, spindle cell lipoma, and cellular angiofibroma); soft tissue tumors with potential for locally aggressive behavior (eg, desmoid fibromatosis); and spindle cell sarcomas (eg, malignant peripheral nerve sheath tumor, dermatofibrosarcoma protuberans, and monophasic synovial sarcoma). The immunohistochemical profile of SFT using conventional markers is relatively nonspecific, with CD34 expression being the most consistent finding reported to date, present in 95% of cases. Other markers that are variably expressed in SFT include CD99, BCL2, nuclear β-catenin, and EMA. However, all these markers are positive in other soft tissue tumors that may mimic SFT.
Several recent studies have detected a recurrent intrachromosomal fusion between the NAB2 and STAT6 genes on chromosome 12 in SFT.11, 12, 13 This genomic inversion at the 12q13 locus results in the formation of a NAB2–STAT6 fusion gene, which can be detected by reverse transcriptase-PCR. However, as these genes are located in close proximity on 12q13, this fusion can only rarely be detected by conventional chromosomal banding or fluorescence in situ hybridization (FISH) analysis. In the study by Robinson et al,11 all 51 SFTs examined harbored the NAB2–STAT6 fusion gene. A lower frequency of this gene fusion was reported in the study by Chmielecki et al,12 being detected in just over 50% of cases, possibly reflecting diagnostic variability. Mohajeri et al13 identified NAB2–STAT6 in 90% of SFTs evaluated. The fusion gene has not been identified in other soft tissue neoplasms studied to date. These results have also been documented in meningeal SFTs (including tumors formerly known as meningeal ‘hemangiopericytoma’).15
STAT6 is a member of the STAT family of cytoplasmic transcription factors, which regulate gene expression by transmitting signals to the nucleus and binding to specific DNA promoter sequences. STAT signaling is critical for normal cellular processes such as embryonic development, innate and adaptive immune function, and regulation of cell differentiation, growth, and apoptosis.18, 19 Activation of STAT family members is a well-recognized alteration in human malignancies.19 STAT6 is composed of a DNA-binding domain, a C-terminal transcriptional activation domain, and a SH2 domain. In contrast to STAT6, NAB2 normally functions as a transcriptional repressor through its interaction with the EGR family of transcription factors and is localized to the nucleus.20 However, NAB2 gains an activation domain when fused to STAT6, and the NAB2–STAT6 fusion gene therefore acts as a transcriptional activator, inducing expression of EGR target genes, which results in increased proliferation.11 It is hypothesized that the fusion protein results in translocation to the nucleus and high levels of expression compared with normal tissues and other neoplasms, as demonstrated by immunofluorescence labeling against the C terminus of STAT611 and immunohistochemical analysis of STAT6 in SFT of the meninges, which showed diffuse nuclear expression in meningeal SFT, but not in other dural tumors.15
We found that 98% of a large cohort of SFTs (including conventional, cellular, atypical, and malignant variants) showed nuclear expression of STAT6. Staining for STAT6 was usually diffuse: 68% of cases showed reactivity for STAT6 in >75% of tumor cells. Further, the intensity of staining was strong in 67% of cases, moderate in 25%, and weak in only 7%. As discussed in the Results section, some tumors showed heterogeneity of staining, both in terms of extent and intensity, which may be because of uneven tissue fixation or loss of antigenicity in older cases for which the unstained slides were stored for extended periods of time. All other tumor types examined were negative for STAT6, except for three dedifferentiated liposarcomas and one deep fibrous histiocytoma, which showed weak staining. Schweizer et al15 reported cytoplasmic expression of STAT6 in tumors other than SFT, albeit usually weak in intensity. In our study, cytoplasmic expression was not detected, which may reflect the higher dilution of antibody used in the current study.
The finding of focal nuclear STAT6 expression in a subset of cases of dedifferentiated liposarcoma is interesting, and may suggest a role for STAT6-mediated transcriptional activity in some cases of dedifferentiated liposarcoma, given that the 12q13 locus contains several different regions that are frequently amplified in dedifferentiated liposarcoma, such as MDM2 and CDK4. In practice, dedifferentiated liposarcoma may enter the morphologic differential diagnosis with SFT, particularly for those tumors occurring in the retroperitoneum, and therefore the finding of STAT6 expression in dedifferentiated liposarcoma is a potential diagnostic pitfall. However, demonstration of positivity for MDM2 and CDK4 by immunohistochemistry21, 22 or MDM2 amplification by FISH23 can help confirm the diagnosis of dedifferentiated liposarcoma.
The discovery of a recurrent gene fusion by whole-exome and transcriptome sequencing is a novel method of detecting translocation-associated sarcomas. In the case of SFT, such methods have resulted in the identification of a useful diagnostic immunohistochemical marker, STAT6, following identification of the recurrent gene fusion product NAB2–STAT6 in this tumor type. This adds to the expanding group of markers for soft tissue tumors identified through molecular genetic methods. For example, gene expression analysis has previously identified ANO1 (anoctamin 1, calcium activated chloride channel; also known as DOG1) as a specific marker of gastrointestinal stromal tumor,24, 25, 26, 27 TLE1 (transducin-like enhancer of split 1) for synovial sarcoma,27, 28 and MUC4 (mucin 4, cell surface associated) for low-grade fibromyxoid sarcoma.29, 30 Nuclear expression of STAT6 is found in nearly all cases of SFT and is very limited in other soft tissue neoplasms. STAT6 is therefore a highly sensitive and almost perfectly specific immunohistochemical marker for SFT, and can be helpful to distinguish this tumor type from histologic mimics.
References
Klemperer P, Rabin CB . Primary neoplasms of the pleura: a report of five cases. Arch Pathol 1931;11:385–412.
Hanau CA, Miettinen M . Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol 1995;26:440–449.
Brunnemann RB, Ro JY, Ordonez NG et al. Extrapleural solitary fibrous tumor: a clinicopathologic study of 24 cases. Mod Pathol 1999;12:1034–1042.
de Saint Aubain Somerhausen N, Rubin BP, Fletcher CD . Myxoid solitary fibrous tumor: a study of seven cases with emphasis on differential diagnosis. Mod Pathol 1999;12:463–471.
Hasegawa T, Matsuno Y, Shimoda T et al. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol 1999;30:1464–1473.
Ng TL, Gown AM, Barry TS et al. Nuclear beta-catenin in mesenchymal tumors. Mod Pathol 2005;18:68–74.
Rao N, Colby TV, Falconieri G et al. Intrapulmonary solitary fibrous tumors: clinicopathologic and immunohistochemical study of 24 cases. Am J Surg Pathol 2013;37:155–166.
Renshaw AA . O13 (CD99) in spindle cell tumors: reactivity with hemangiopericytoma, solitary fibrous tumor, synovial sarcoma, and meningioma but rarely with sarcomatoid mesothelioma. Appl Immunohistochem 1995;3:250–256.
Suster S, Fisher C, Moran CA . Expression of bcl-2 oncoprotein in benign and malignant spindle cell tumors of soft tissue, skin, serosal surfaces, and gastrointestinal tract. Am J Surg Pathol 1998;22:863–872.
Chilosi M, Facchettti F, Dei Tos AP et al. Bcl-2 expression in pleural and extrapleural solitary fibrous tumours. J Pathol 1997;181:362–367.
Robinson DR, Wu YM, Kalyana-Sundaram S et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 2013;45:180–185.
Chmielecki J, Crago AM, Rosenberg M et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet 2013;45:131–132.
Mohajeri A, Tayebwa J, Collin A et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, non-random secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer 2013;52:873–886.
Calonje E, Fletcher CDM, Hemangiopericytoma, In: Fletcher CD, (ed). Diagnostic Histopathology of Tumors 3rd edn. Churchill Livingstone/Elsevier: Edinburgh, UK, 2007, pp 72–74.
Schweizer L, Koelsche C, Sahm F et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol 2013;125:651–658.
Fletcher CDM, Bridge JA, Lee J-C . Extrapleural solitary fibrous tumour In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, (eds). WHO Classification of Tumours of Soft Tissue and Bone. IARC: Lyon, France, 2013, pp 80–82.
Demicco EG, Park MS, Araujo DM et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol 2012;25:1298–1306.
Bromberg J, Darnell JE Jr. . The role of STATs in transcriptional control and their impact on cellular function. Oncogene 2000;19:2468–2473.
Buettner R, Mora LB, Jove R . Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res 2002;8:945–954.
Kumbrink J, Kirsch KH, Johnson JP . EGR1, EGR2, and EGR3 activate the expression of their coregulator NAB2 establishing a negative feedback loop in cells of neuroectodermal and epithelial origin. J Cell Biochem 2010;111:207–217.
Binh MB, Sastre-Garau X, Guillou L et al. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol 2005;29:1340–1347.
Dei Tos AP, Doglioni C, Piccinin S et al. Coordinated expression and amplification of the MDM2, CDK4, and HMGI-C genes in atypical lipomatous tumours. J Pathol 2000;190:531–536.
Sandberg AA . Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: liposarcoma. Cancer Genet Cytogenet 2004;155:1–24.
Espinosa I, Lee CH, Kim MK et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol 2008;32:210–218.
Miettinen M, Wang ZF, Lasota J . DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol 2009;33:1401–1408.
West RB, Corless CL, Chen X et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 2004;165:107–113.
Baird K, Davis S, Antonescu CR et al. Gene expression profiling of human sarcomas: insights into sarcoma biology. Cancer Res 2005;65:9226–9235.
Nielsen TO, West RB, Linn SC et al. Molecular characterisation of soft tissue tumours: a gene expression study. Lancet 2002;359:1301–1307.
Moller E, Hornick JL, Magnusson L et al. FUS-CREB3L2/L1-positive sarcomas show a specific gene expression profile with upregulation of CD24 and FOXL1. Clin Cancer Res 2011;17:2646–2656.
Doyle LA, Moller E, Dal Cin P et al. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am J Surg Pathol 2011;35:733–741.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Doyle, L., Vivero, M., Fletcher, C. et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 27, 390–395 (2014). https://doi.org/10.1038/modpathol.2013.164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2013.164
Keywords
This article is cited by
-
Solitary fibrous tumor occurring in the colon as submucosal mesenchymal lesion: report of two cases and review of the literature
Clinical Journal of Gastroenterology (2024)
-
A giant orbital solitary fibrous tumor treated by surgical excision: a case report and literature review
Diagnostic Pathology (2023)
-
Large Solitary Fibrous Tumor (SFT) of the penis- a case report and review of literature
BMC Urology (2023)
-
A Rare Case of Solitary Fibrous Tumor of Maxilla: Findings on F-18 FDG and Ga-68 DOTANOC PET-CT
Nuclear Medicine and Molecular Imaging (2023)
-
Histopathologic Diagnosis of Sinonasal Tumors: Challenges and the Importance of Establishing the Correct Diagnosis
Current Otorhinolaryngology Reports (2023)