Abstract
Aurora-A is a mitotic kinase implicated in oncogenesis and is known to be overexpressed in B-cell lymphomas and plasma cell myeloma. The expression of Aurora-A kinase (henceforth referred to as Aurora-A) in T-cell lymphomas is not well characterized. In this study, we assessed Aurora-A expression by immunohistochemical analysis in 100 lymphomas encompassing a variety of T-cell lymphomas as categorized in the World Health Organization classification. Aurora-A expression was highest in anaplastic large-cell lymphomas and variably expressed in other types of T-cell lymphomas. In addition, the pattern of Aurora-A expression was predominantly cytoplasmic in ALK-positive anaplastic large-cell lymphoma and was nuclear in ALK-negative anaplastic large-cell lymphoma and other T-cell lymphomas, suggesting altered biochemical mechanisms of Aurora-A nuclear transport in ALK-positive anaplastic large-cell lymphoma. Reverse transcriptase-PCR analysis showed that Aurora-A is more highly expressed in ALK-positive anaplastic large-cell lymphoma than in ALK-negative anaplastic large-cell lymphoma, and is relatively lower in peripheral T-cell lymphomas. Using western blot analysis and the DEL cell line (derived from ALK-positive anaplastic large-cell lymphoma), we showed that Aurora-A expression is decreased after treatment with either MYC or MEK inhibitors, consistent with the MYC and MAP kinase signaling pathways being involved in driving Aurora-A expression; the greatest decrease was observed after MYC inhibition. These findings provide insights into the possible importance of Aurora-A overexpression in anaplastic large-cell lymphoma pathogenesis, and also suggest that Aurora-A inhibition could be a potential therapeutic approach for patients with anaplastic large-cell lymphoma.
Similar content being viewed by others
Main
Aurora kinases are a family of serine/threonine kinases that play a key role in regulating the cell cycle. Three different classes of aurora kinases, A, B and C, have been identified,1 each of which is involved in different mechanisms and aspects of cell signaling and mitotic division.2 Aurora-A kinase has been implicated in several biochemical pathways and has been suggested to have roles as both an oncogene and a tumor suppressor.3 Overexpression of Aurora-A has been shown in a variety of solid tumors arising in the breast, pancreas, gastrointestinal carcinomas at various sites, bladder, prostate, ovary and central nervous system, and has been implicated in their tumorigenesis.4, 5, 6, 7 Aurora-A overexpression has also been implicated in the pathogenesis of a variety of hematologic neoplasms including chronic lymphocytic leukemia/small lymphocytic lymphoma,8 highly aggressive B-cell non-Hodgkin’s lymphomas9, 10 and most recently in natural killer cell lymphomas.11 Expression of Aurora-A kinase has been associated with inferior survival in patients with plasma cell myeloma.12
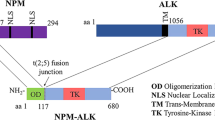
T-cell lymphomas represent approximately 10% of all non-Hodgkin’s lymphomas. Because of their broad morphological spectrum, immunophenotypic variations, and absence of recurrent genetic abnormalities, the pathogenesis of most T-cell lymphomas is poorly understood. Anaplastic lymphoma kinase (ALK)-positive anaplastic large-cell lymphoma, however, is characterized by chromosomal translocations, or rarely inversions, that involve the ALK gene at chromosome locus 2p23.13, 14, 15, 16
In this study, we assessed Aurora-A protein expression by using immunohistochemistry in a variety of T-cell lymphoma types. After showing high Aurora-A expression in anaplastic large-cell lymphoma, we used reverse transcriptase-PCR (RT-PCR) to semiquantify Aurora-A expression and performed in vitro experiments using western blot analysis and an ALK-positive anaplastic large-cell lymphoma cell line. These results show high Aurora-A expression in ALK-positive anaplastic large-cell lymphoma, driven, at least in part, by the MYC and MAP kinase signaling pathways.
Materials and methods
Case Selection
A total of 100 cases encompassing the spectrum of T-cell lymphomas as described in the 2008 World Health Organization (WHO) classification scheme were included in this study. The study group included 22 ALK-negative anaplastic large-cell lymphomas, 15 ALK-positive anaplastic large-cell lymphoma, 14 peripheral T-cell lymphoma not otherwise specified, 13 cutaneous anaplastic large-cell lymphoma, 7 angioimmunoblastic T-cell lymphoma, 6 extranodal NK/T cell lymphoma, nasal type, 6 enteropathy-associated T-cell lymphoma, 6 mycosis fungoides, 5 T-lymphoblastic lymphoma/leukemia (with lymph node or extranodal sites of disease), 3 T-prolymphocytic leukemia and 3 subcutaneous panniculitis-like T-cell lymphoma. In addition, 5 cases of reactive follicular hyperplasia were assessed, including 3 lymph nodes and 2 tonsils.
Aurora-A Immunohistochemical Staining and Grading
Immunohistochemical analysis was performed using fixed, paraffin-embedded tissue sections. A mouse monoclonal anti-human Aurora-A antibody was used (Bethyl Labs, Montgomery, TX, USA). After overnight drying of the sections in (60 °C) oven, immunohistochemical analysis was performed using the procedure for the DAKO Autostainer (DAKO, Carpinteria, CA, USA). Any cytoplasmic and/or nuclear staining was considered positive. Staining of endothelial cell or macrophage nuclei served as an internal control. Each case was semiquantitatively estimated for the percentage of positive cells (0–25%; 25–50%; >50%) as well as staining intensity (1–3+). The criteria used for assessing intensity of Aurora-A staining were as follows: 2+ was considered equivalent to the intensity of staining of reactive cells in benign tonsils; staining that was weaker or stronger than cells in benign tonsils were considered 1+ and 3+, respectively.
Quantitative Real-Time RT-PCR for Aurora-A mRNA Expression
Aurora-A mRNA expression was assessed by real-time quantitative RT-PCR in 20 specimens including 9 cases of peripheral T-cell lymphoma not otherwise specified, 3 cases of ALK-positive anaplastic large-cell lymphoma, 4 cases of ALK-negative anaplastic large-cell lymphoma and 4 benign tissues. Total mRNA was extracted under RNase free conditions from paraffin blocks of tumor tissues. The RecoverAll Total Nucleic Acid Isolation Kit (Ambion, Austin, TX, USA) with glass fiber-filter methodology for RNA extraction was used. RNA quality and quantity was evaluated by ultraviolet light absorbance on a spectrophotometer. cDNA was prepared using SuperScript III Reverse Transcriptase kit (Invitrogen, Madison, WI, USA) as per the manufacturer’s instructions. Real-time PCR amplification and analysis was performed using Rotor Gene (Qiagen, Valencia, CA, USA) and LightCycler 480 (Roche Applied Science, Indianapolis, IN, USA) instruments. The β2-microglobulin was used as housekeeping gene for verification and quantitation of amplifiable RNA. The PCR master mix included forward (5′-GCAGATTTTGGGTGGTCAGT-3′) and reverse (5′-CAAAAGGAGGCTTCCCAACT-3′) primers and probe (HYB(FAM-6) 5′-AATGATTGAAGGTCGGATGC-3′), each at 10 μM. mRNA expression of tumors was compared with benign lymph node and tonsil tissues using the ΔΔCT method. Statistical analysis (described below) was performed comparing high expression versus low or absent expression of Aurora-A kinase normalized to β2-microglobulin.
Cell Culture and Western Blot Analysis
DEL (ALK-positive anaplastic large-cell lymphoma) cells were cultured at 37 °C in a 5% CO2 atmosphere in RPMI-1640 media. For MEK and MYC inhibitor experiments, 1.0 ml of a log phase DEL cell suspension was added to 4.0 ml of fresh culture media in 60 mm dishes. Then, 5 μl of 25 mM stock solutions of 10058-F4 MYC inhibitor (Sigma-Aldrich) or PD98059 MEK-1 inhibitor (Cell Signaling Technologies) in DMSO were added to 2, 6 and 24 h treatment dishes to a final concentration of 25 nM. To 0 h control dishes, 5 μl of DMSO alone was added.
After completion of inhibitor treatment, the above DEL cell cultures were pelleted, re-suspended in cold DPBS and re-pelleted. Lysates were prepared by re-suspending cell pellets in lysate buffer consisting of RIPA (Thermo Scientific, Rockford, IL, USA) containing 1 μM dithiothreitol and protease inhibitors (16 μg/ml aprotinin, 1 μg/ml each of leupeptin A, pepstatin and chymostatin (Sigma, St Louis, MO, USA) and 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (MP Biomedicals, Solon, OH, USA)). Cell suspensions were centrifuged at 16 100 g at 4 °C for 20 min and the supernatant collected, and the centrifugation repeated. Additionally, cytoplasmic compartment lysates were prepared from untreated log phase DEL cells and the nuclei were isolated through 1 mM sucrose as previously described.17
Lysate total protein was determined using the Pierce BCA method (Thermo Scientific). Lysates (15 μg total protein each) were electrophoresed on 10% polyacrylamide gels and electrotransferred to Imobilon PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blocked with 4% dried milk in TBST and incubated with clone 35C1 mouse monoclonal anti-human Aurora-A (1:500, ab13824; Abcam), or mouse monoclonal anti-β-actin (1:4000, A2228; Sigma) for 90 min at room temperature. Blots hybridized with anti-human phospho-Thr288-Aurora-A (1:500, ab58494; Abcam ) were incubated in 5% BSA in TBST at 4 °C overnight. Secondary antibodies were goat anti-mouse or goat anti-rabbit IgG-HRP (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) incubated for 30 min. Blots were developed with Pierce ECL (Thermo Scientific) and exposed to X-ray film. Relative ratios of Aurora-A protein signal to β-actin signal within samples were determined by densitometry using NIH ImageJ software.
Statistical Analysis
Using SAS software, Fisher’s exact and χ2 tests (Mantel–Haenszel) were used to determine the association between various lymphoma categories and Aurora-A immunohistochemical staining pattern, intensity and quantity. A P-value of <0.05 was considered statistically significant. For the real-time RT-PCR analysis, normalized ΔCt values were calculated for each lymphoma category.
Results
Immunohistochemical Findings
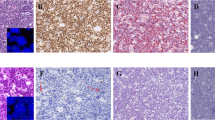
Aurora-A was detected in 68 of 100 (68%) cases of T-cell lymphoma (Table 1). Aurora-A was expressed in 13 of 15 (87%) cases of ALK-positive anaplastic large-cell lymphoma and in 15 of 22 (68%) ALK-negative anaplastic large-cell lymphoma. Other types of T-cell lymphoma in which a majority of cases expressed Aurora-A included all 14 (100%) cases of peripheral T-cell lymphoma not otherwise specified; 9 of 13 (69%) cutaneous anaplastic large-cell lymphoma; 4 of 6 (67%) mycosis fungoides, 2 of 3 (67%) T-cell prolymphocytic leukemia and 3 of 5 (60%) T-cell lymphoblastic lymphoma/leukemia. Aurora-A was less frequently expressed by extranodal NK/T-cell lymphoma, nasal type (3 of 6; 50%), angioimmunoblastic T-cell lymphoma (3 of 7; 43%), subcutaneous panniculitis-like T-cell lymphoma (1 of 3; 33%) and enteropathy-associated T-cell lymphoma (1 of 6; 17%).
For each lymphoma type, the percentage of positive cells was estimated, the pattern of Aurora-A staining was categorized as nuclear versus cytoplasmic and the intensity of staining was determined (Table 1). Greater than 50% of tumor cells were positive for Aurora-A in 3 of 4 (75%) mycosis fungoides, 8 of 14 (57%) peripheral T-cell lymphoma not otherwise specified, 6 of 13 (46%) ALK-positive anaplastic large-cell lymphoma, 4 of 8 (33.3%) ALK-negative anaplastic large-cell lymphoma, 3 of 9 (33%) cutaneous anaplastic large-cell lymphoma, 1 of 3 (33%) extranodal NK/T-cell lymphoma, nasal type and 1 of 7 (14%) angioimmunoblastic T-cell lymphoma. All other T-cell lymphomas had a lesser number of Aurora-A positive cells (between 25 and 50%) or were negative.
Differences in subcellular distribution of Aurora-A were observed in anaplastic large-cell lymphoma. All cases of ALK-positive anaplastic large-cell lymphoma showed cytoplasmic expression of Aurora-A. In contrast, 67% of cases of ALK-negative anaplastic large-cell lymphoma showed nuclear Aurora-A expression, with only 33% of cases having a cytoplasmic pattern (P<0.001). Two other lymphoma categories showed a small subset of cases with cytoplasmic Aurora-A expression: 4 of 14 (28.5%) peripheral T-cell lymphoma not otherwise specified (Figure 1) and 1 of 7 (14.3%) angioimmunoblastic T-cell lymphoma. However, all other types of T-cell lymphomas showed nuclear expression of Aurora-A. The differences in subcellular distribution of Aurora-A between ALK-positive anaplastic large-cell lymphoma versus ALK-negative anaplastic large-cell lymphoma (P<0.001), ALK-positive anaplastic large-cell lymphoma versus peripheral T-cell lymphoma not otherwise specified (P<0.001) and ALK-positive anaplastic large-cell lymphoma versus cutaneous anaplastic large cell lymphoma (P<0.001) reached statistical significance (Table 1).
In tumors that were positive for Aurora-A expression, 2+ or 3+ staining intensity was highest in peripheral T-cell lymphoma not otherwise specified (8 of 14; 57%), followed by cutaneous anaplastic large-cell lymphoma (5 of 9; 56%), ALK-positive anaplastic large-cell lymphoma (7 of 13; 54%), ALK-negative anaplastic large-cell lymphoma (4 of 15; 26%), extranodal NK/T-cell lymphoma of nasal type (1 of 6; 17%), mycosis fungoides (1 of 6; 17%), angioimmunoblastic T-cell lymphoma (1 of 7; 14%) and no cases of T-lymphoblastic leukemia/lymphoma. In addition, almost all cases of ALK-positive ALCL had 3+ staining that was less common in other T-cell lymphomas.
Quantitative Real-Time RT-PCR for Aurora-A mRNA Expression
Using RT-PCR, Aurora-A mRNA transcript levels were highest in ALK-positive anaplastic large-cell lymphoma (mean copy number, 1.57) compared with ALK-negative anaplastic large-cell lymphoma (mean, 0.9), peripheral T-cell lymphoma not otherwise specified (mean, 0.55) and benign lymphoid tissues (mean, 0.57; Figure 2).
Western Blot Analysis of Aurora-A in DEL Cells
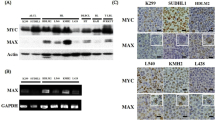
To confirm our immunohistochemical findings for Aurora-A cytoplasmic localization in ALK-positive anaplastic large-cell lymphoma, lysates were prepared from nuclear and cytoplasmic compartments of cultured DEL (ALK+ anaplastic large-cell lymphoma) cells. Consistent with the immunohistochemical results, Aurora-A protein was detected only in the cytoplasmic compartment of DEL cells by western blotting (Figure 3a, left panel). We also blotted these nuclear and cytoplasmic lysates for phosphorylated Aurora-A. Detection of phosphorylated Aurora-A required longer exposure of the ECL reaction to the X-ray film; however, phosphosphorylated Aurora-A was found to be expressed in approximately equal amounts in the cytoplasmic and nuclear compartments (Figure 3a, right panel). Interestingly, the slower migrating phospho-Aurora-A species detected in the cytoplasmic compartment was seen at much lower levels in the nuclear compartment, suggesting that nuclear localization of Aurora-A may be related to differential phosphorylation states.
(a) Aurora-A and phospho-Thr288-Aurora-A protein expression in cytoplasmic and nuclear compartments of DEL cell line (ALK-positive anaplastic large-cell lymphoma) assessed by western blotting. (b) DEL cells were treated with a MEK-1 or MYC inhibitor for various lengths of time. Total cell lysates were then prepared and subjected to western blotting for Aurora-A. Aurora-A expression levels decreased after treatment with the inhibitors, suggesting that Aurora An expression is mediated by the MAP kinase and MYC signaling pathways. (c) Quantification of western blot data shown in (b).
We also treated DEL cells with inhibitors of the MYC and MAP kinase signaling pathways for 2–24 h. Although the effect of MYC inhibition was most striking, in both cases Aurora-A protein expression began to decrease after 6 h of inhibitor treatment and was markedly decreased after 24 h of treatment (Figure 3b and c).
Discussion
Aurora-A is a serine/threonine kinase that controls spindle assembly, centrosome maturation and progression of the cell cycle from G2 to M phase.2 Overexpression of Aurora-A has been implicated in the pathogenesis of several solid tumors, B-cell lymphomas and plasma cell myeloma, but little information related to Aurora-A is available in T-cell lymphomas.
Using RT-PCR, we have shown quantitative differences in the levels of Aurora-A mRNA transcripts in ALK-positive anaplastic large-cell lymphoma, ALK-negative anaplastic large-cell lymphoma and peripheral T-cell lymphoma not otherwise specified. The differences in expression levels of Aurora-A mRNA between ALK-negative anaplastic large-cell lymphoma and peripheral T-cell lymphoma not otherwise specified may have pathogenic implications. Clinicopathologic distinction of ALK-negative anaplastic large-cell lymphoma and peripheral T-cell lymphoma not otherwise specified with many CD30-positive cells can be challenging. In this study, we used morphologic and immunohistochemical findings to identify ALK-negative anaplastic large-cell lymphoma. Cases of ALK-negative anaplastic lymphoma kinase frequently showed preferential sinusoidal infiltration by neoplastic cells, tumor cells with horseshoe-shaped nuclei (so-called hallmark cells), strong and uniform CD30 expression, a cytotoxic immunophenotype and clusterin expression.18 Although, currently there is no difference in management between patients with ALK-negative anaplastic large-cell lymphoma versus peripheral T-cell lymphoma not otherwise specified, Savage et al19 have shown that there are significant differences in failure free and overall survival rates between patients with ALK-negative anaplastic large-cell lymphoma and peripheral T-cell lymphoma not otherwise specified. Furthermore, comparative genomic hybridization analysis of peripheral T-cell lymphoma not otherwise specified and ALK-negative anaplastic large-cell lymphoma have also shown significant differences in genetic alterations. Loss of chromosomes 9p21-pter and 5q are frequent in peripheral T-cell lymphoma not otherwise specified compared with ALK-negative anaplastic large-cell lymphoma, which more frequently has gains in chromosome 1q.20 In our study, we found Aurora-A mRNA transcript levels by RT-PCR in ALK-negative anaplastic large-cell lymphoma to be at least 1.67-fold higher than peripheral T-cell lymphoma not otherwise specified. Additionally, Aurora-A mRNA levels were not significantly elevated in peripheral T-cell lymphoma not otherwise specified, being only marginally different from benign tissues. These data suggest possible differences between ALK-negative anaplastic large-cell lymphoma and peripheral T-cell lymphoma not otherwise specified at the molecular and biologic levels, although they can exhibit morphologic and immunophenotypic overlap.
Using immunohistochemical analysis, we found that Aurora-A is expressed in an appreciable subset of most types of T-cell lymphomas, with expression levels being highest in anaplastic large-cell lymphoma, and least common and at a low level in enteropathy-associated T-cell lymphoma. Among anaplastic large-cell lymphomas, ALK-positive cases had the highest expression of Aurora-A, shown by a greater percentage of positive cells and higher intensity of staining shown by immunohistochemistry, as well as highest Aurora-A mRNA transcript levels by RT-PCR. Immunohistochemical analysis also showed that Aurora-A is consistently expressed in a cytoplasmic pattern in ALK-positive anaplastic large-cell lymphoma, whereas it is expressed in a nuclear pattern in most types of T-cell lymphomas, and Aurora-A is localized to the centrosomes in the nucleus in most phases of cell cycle. Therefore, the cytoplasmic pattern of Aurora-A expression in ALK-positive anaplastic large-cell lymphoma is aberrant. Diffuse cytoplasmic staining for Aurora-A has also been shown in many solid tumors, such as carcinomas arising in the breast, ovary, colon and rectum. Cytoplasmic expression has been attributed primarily to aberrant phosphorylation of cytoplasmic proteins.6, 21, 22, 23 Indeed, we found subtle differences in phosphorylated Aurora-A species in DEL cells by western blot analysis. Aurora-A overexpression and differential subcellular localization in ALK-positive anaplastic large-cell lymphoma compared with other types of T-cell lymphomas may provide insight into the pathogenesis. It can be hypothesized that biochemical mechanisms of Aurora-A localization and protein binding partners may be dysregulated, in addition to downstream targets of Aurora-A signaling. In aggregate, high levels of Aurora-A expression and its aberrant pattern implicate Aurora-A as being involved in the pathogenesis of ALK-positive anaplastic large-cell lymphoma. Furthermore, the absence of chromosome 20q alterations in ALK-positive anaplastic large-cell lymphoma, unlike NK-cell lymphomas that show frequent amplifications of 20q13,24 might imply that Aurora-A itself may not be oncogenic by itself, but in co-operation with the upstream or downstream targets.
It is known that novel ALK fusion genes, most often NPM-ALK, are necessary but not sufficient to cause ALK-positive anaplastic large-cell lymphoma. Therefore, other ‘second hits’ are required for oncogenesis. One pathway implicated in the pathogenesis of ALK-positive anaplastic large-cell lymphoma is the RAS/RAF/MEK/MAPK pathway.15 As shown in the studies on primary rat embryonic cells, NPM-ALK cooperates with RAS for oncogenic transformation.25 NPM-ALK activates RAS and phosphorylates downstream components of the mitogen-activated protein kinase 1 (MAPK1) signaling pathway, leading to activation of T lymphocytes.26 The phosphorylation of MAPK and mitogen-induced extracellular kinase (MEK), a direct activator of MAPK by NPM-ALK, is shown to be independent of Raf.27 As Aurora-A has been shown to be a direct downstream target of the MAPK pathway in pancreatic cancer cells,28 it seems reasonable to hypothesize that activation of the RAS/RAF/MEK/MAPK pathway may be responsible for Aurora-A overexpression in ALK-positive anaplastic large-cell lymphoma.2 In vitro experiments using a MEK-1 inhibitor, PD98059, and DEL ALK-positive anaplastic large-cell lymphoma cells support the hypothesis as treatment of DEL cells with PD98059 resulted in decreased Aurora-A expression.
MYC plays a pathogenic role in ALK-positive anaplastic large-cell lymphoma. As demonstrated in Rat 1a fibroblasts, NPM-ALK induces its tumorigenic effect by partially activating MYC.29 NPM-ALK phosphorylates the RAS/RAF/MAPK pathway, thereby activating the MYC gene promoter.30 In support of this, MYC is overexpressed specifically in ALK-positive anaplastic large-cell lymphoma and is considered to be a downstream target of aberrant ALK signaling.31 MYC and Aurora-A also cooperate in tumorigenesis.32 MYC upregulates Aurora-A expression through activation of E2F.3, 33 Additionally, Aurora-A overexpression enhances expression and transcription of MYC.32 Telomerase activity in ovarian and breast carcinoma cells by Aurora-A has been shown to be mediated by increased MYC expression.34 Therefore, a positive feedback loop between MYC and Aurora-A expression may exist. In accordance with these data, we performed in vitro experiments using DEL cells and a MYC inhibitor, 10058-F4. We showed a faster and greater decrease in Aurora-A expression with MYC inhibition, rather than MEK inhibition, consistent with a more direct effect of MYC on Aurora-A expression.
In summary, we have shown that Aurora-A is expressed in a subset of cases within a number of different types of T-cell lymphomas. The highest levels of Aurora-A expression were observed in ALK-positive anaplastic large-cell lymphoma. ALK-positive anaplastic large-cell lymphoma also showed a consistent aberrant cytoplasmic pattern of expression, compared with other types of T-cell lymphomas. In vitro experiments using the DEL cell line showed decreased Aurora-A expression after treatment with either MEK or MYC inhibitors, suggesting that the RAS/RAF/MEK/MAPK and MYC pathways are involved in Aurora-A expression, in accord with data derived from solid tumor models in the literature. In aggregate, these data provide some insights into the possible importance of Aurora-A expression in ALK-positive anaplastic large-cell lymphoma. The data also suggest that Aurora-A inhibitors could be potentially useful in clinical trials to treat patients with ALK-positive anaplastic large-cell lymphoma.
References
Nigg EA . Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol 2001;2:21–32.
Fu J, Bian M, Jiang Q et al. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res 2007;5:1–10.
Lehman NL, O’Donnell JP, Whiteley LJ et al. Aurora-A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle 2012;11:489–502.
Tong T, Zhong Y, Kong J et al. Overexpression of Aurora-A contributes to malignant development of human esophageal squamous cell carcinoma. Clin Cancer Res 2004;10:7304–7310.
Kamada K, Yamada Y, Hirao T et al. Amplification/overexpression of Aurora-A in human gastric carcinoma: potential role in differentiated type gastric carcinogenesis. Oncol Rep 2004;12:593–599.
Marumoto T, Zhang D, Saya H . Aurora-A – a guardian of poles. Nat Rev Cancer 2005;5:42–50.
Lehman NL, Tibshirani R, Hsu JY et al. Oncogenic regulators and substrates of the anaphase promoting complex/cyclosome are frequently overexpressed in malignant tumors. Am J Pathol 2007;170:1793–1805.
Inamdar KV, O’Brien S, Sen S et al. Aurora-A kinase nuclear expression in chronic lymphocytic leukemia. Mod Pathol 2008;21:1428–1435.
Yakushijin Y, Hamada M, Yasukawa M . The expression of the aurora-A gene and its significance with tumorgenesis in non-Hodgkin’s lymphoma. Leuk Lymphoma 2004;45:1741–1746.
Hamada M, Yakushijin Y, Ohtsuka M et al. Aurora2/BTAK/STK15 is involved in cell cycle checkpoint and cell survival of aggressive non-Hodgkin’s lymphoma. Br J Haematol 2003;121:439–447.
Iqbal J, Weisenburger D, Chowdhury A et al. Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic γδ T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro. Leukemia 2011;25:348–358.
Hose D, Rème T, Meissner T et al. Inhibition of aurora kinases for tailored risk-adapted treatment of multiple myeloma. Blood 2009;113:4331–4340.
Stein H, Foss HD, Durkop H et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood 2000;96:3681–3695.
Kinney MC, Higgins RA, Medina EA . Anaplastic large cell lymphoma: twenty-five years of discovery. Arch Pathol Lab Med 2011;135:19–43.
Tabbo F, Barreca A, Piva R et al. ALK signaling and target therapy in anaplastic large cell lymphoma. Front Oncol 2012;2:41.
Medeiros LJ, Elenitoba-Johnson KS . Anaplastic large cell lymphoma. Am J Clin Pathol 2007;127:707–722.
Filiano AJ, Bailey CD, Tucholski J et al. Transglutaminase 2 protects against ischemic insult, interacts with HIF1beta, and attenuates HIF1 signaling. FASEB J 2008;22:2662–2675.
Saffer H, Wahed A, Rassidakis GZ et al. Clusterin expression in malignant lymphomas: a survey of 266 cases. Mod Pathol 2002;15:1221–1226.
Savage KJ, Harris NL, Vose JM et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 2008;111:5496–5504.
Zettl A, Rüdiger T, Konrad MA et al. Genomic profiling of peripheral T-cell lymphoma, unspecified, and anaplastic large T-cell lymphoma delineates novel recurrent chromosomal alterations. Am J Pathol 2004;164:1837–1848.
Takahashi T, Futamura M, Yoshimi N et al. Centrosomal kinases, HsAIRK1 and HsAIRK3, are overexpressed in primary colorectal cancers. Jpn J Cancer Res 2000;91:1007–1014.
Tanaka T, Kimura M, Matsunaga K et al. Centrosomal kinase AIK1 is overexpressed in invasive ductal carcinoma of the breast. Cancer Res 1999;59:2041–2044.
Gritsko TM, Coppola D, Paciga JE et al. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res 2003;9:1420–1426.
Iqbal J, Kucuk C . Genomic analyses reveal global functional alterations that promote tumor growth and novel tumor suppressor genes in natural killer-cell malignancies. Leukemia 2009;23:1139–1151.
Simonitsch I, Polgar D, Hajek M et al. The cytoplasmic truncated receptor tyrosine kinase ALK homodimer immortalizes and cooperates with ras in cellular transformation. FASEB J 2001;15:1416–1418.
Turner SD, Yeung D, Hadfield K et al. The NPM-ALK tyrosine kinase mimics TCR signalling pathways, inducing NFAT and AP-1 by RAS-dependent mechanisms. Cell Signal 2007;19:740–747.
Marzec M, Kasprzycka M, Liu X et al. Oncogenic tyrosine kinase NPM/ALK induces activation of the MEK/ERK signaling pathway independently of c-Raf. Oncogene 2006;26:813–821.
Furukawa T, Kanai N, Shiwaku H et al. AURKA is one of the downstream targets of MAPK1/ERK2 in pancreatic cancer. Oncogene 2006;25:4831–4839.
Wellmann A, Doseeva V, Butscher W et al. The activated anaplastic lymphoma kinase increases cellular proliferation and oncogene up-regulation in rat 1a fibroblasts. FASEB J 1997;11:965–972.
Amin HM, Lai R . Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood 2007;110:2259–2267.
Raetz EA, Perkins SL, Carlson MA et al. The nucleophosmin-anaplastic lymphoma kinase fusion protein induces c-Myc expression in pediatric anaplastic large cell lymphomas. Am J Pathol 2002;161:875–883.
Yang S, He S, Zhou X et al. Suppression of Aurora-A oncogenic potential by c-Myc downregulation. Exp Mol Med 2010;42:759.
den Hollander J, Rimpi S, Doherty JR et al. Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood 2010;116:1498–1505.
Yang H, Ou CC, Feldman RI et al. Aurora-A kinase regulates telomerase activity through c-Myc in human ovarian and breast epithelial cells. Cancer Res 2004;64:463–467.
Acknowledgements
We thank Ms Lisa J Whiteley, Ms Kathleen M Roszka and Ms Caitlin Williams for their technical assistance. This work was supported by the CAP Research Grant (to RK-S) and NIH Grant K08 NS45077 (to NLL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kanagal-Shamanna, R., Lehman, N., O'Donnell, J. et al. Differential expression of aurora-A kinase in T-cell lymphomas. Mod Pathol 26, 640–647 (2013). https://doi.org/10.1038/modpathol.2012.211
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.211
Keywords
This article is cited by
-
Aurora-A kinase is differentially expressed in the nucleus and cytoplasm in normal Müllerian epithelium and benign, borderline and malignant serous ovarian neoplasms
Diagnostic Pathology (2021)
-
The Future of Combination Therapies for Peripheral T Cell Lymphoma (PTCL)
Current Hematologic Malignancy Reports (2018)
-
Therapeutic options in peripheral T cell lymphoma
Journal of Hematology & Oncology (2016)
-
New developments in the pathology of malignant lymphoma: a review of the literature published from January 2013 to April 2013
Journal of Hematopathology (2013)