Abstract
Chromophobe renal cell carcinoma (RCC) is a form of renal cancer that may be confused with other eosinophilic renal tumors, including oncocytoma, type 2 papillary RCC, and clear-cell RCC with eosinophilic features. There are currently no robust markers to distinguish these neoplasms. Chromophobe RCC and renal oncocytoma are presumably derived from the distal nephron. FXYD2 is a distal tubule regulator of the trimeric Na+/K+-transporting ATPase that is enriched in kidney tissue. In this study, we investigated the expression of FXYD2 in normal human kidney, 27 chromophobe RCCs, 30 oncocytomas, 15 clear-cell RCCs, and 11 papillary RCCs. Immunohistochemical staining for FXYD2 showed diffuse, strong immunoreactivity in the basolateral membrane of distal tubules of normal human kidney. Ninety-six percent (26/27) of chromophobe RCCs were immunoreactive for FXYD2 in a distinctly membranous pattern. Twenty-five of these tumors showed at least focal 2+ staining. In contrast, only 17% (5/30) of renal oncocytomas, 11% (2/15) of clear-cell RCCs, and 0% (0/11) of papillary RCCs displayed FXYD2 immunoreactivity. None of these cases showed ≥2+ FXYD2 staining. A subset of cases was confirmed as oncocytoma or chromophobe RCC using cytokeratin 7, colloidal iron, and interphase fluorescence in situ hybridization analysis of chromosomes 1, 2, 6, 10, and 17. Among this subset, 100% (7/7) of chromophobe RCCs were FXYD2 positive, whereas 17% (2/12) of oncocytomas were stained with FXYD2. The oncocytomas that stained with FXYD2 did so in a weak (1+), patchy manner. In contrast, chromophobe RCCs showed ≥2+ staining in 86% (6/7) of these tumors. For comparison, this subset was also stained for kidney-specific cadherin (Ksp-cadherin). Ksp-cadherin showed positive staining in 100% (7/7) of chromophobe RCCs and 33% (4/12) of oncocytomas. This is the first report demonstrating the potential utility of FXYD2 immunohistochemistry in the diagnosis of chromophobe RCC.
Similar content being viewed by others
Main
Renal cell carcinomas (RCCs) are among the most deadly forms of genitourinary malignancies. Approximately 20–30% of patients present with metastatic disease.1 Worldwide, 270 000 individuals were diagnosed with RCC in 2008. In all, 116 000 of these patients died as a result of their disease.2 In contrast, renal oncocytomas are benign tumors of the kidney with a favorable prognosis. Accurate diagnosis is essential for guiding appropriate clinical management.
RCCs are classified by histology into various subtypes, including conventional clear-cell, papillary, and chromophobe among others. Multiple studies have shown that chromophobe RCCs without sarcomatoid differentiation have a more favorable prognosis.3, 4 However, recent investigations have demonstrated equivalent prognosis between chromophobe RCC and conventional clear-cell RCC when tumors of equivalent stage are compared.5, 6 Pathological tumor stage, tumor necrosis, and sarcomatoid change correlate with chromophobe RCC prognosis.7
The benign renal oncocytoma is a histological mimic of chromophobe RCC, particularly its eosinophilic variant. Other eosinophilic renal tumors also may histologically mimic chromophobe RCC, including type 2 papillary RCC and clear-cell RCC with eosinophilic features.8 Discriminating such tumors is not an infrequent diagnostic dilemma to the practicing surgical pathologist. Given the marked difference in prognosis between chromophobe RCC and oncocytoma, it is critical to distinguish between them.
A variety of ancillary strategies have been explored to assist pathologists in distinguishing chromophobe RCCs from oncocytomas, including immunohistochemistry, Hale’s colloidal iron histochemistry, electron microscopy, cytogenetics, and fluorescence in situ hybridization (FISH).9, 10, 11, 12, 13, 14 However, none of these modalities has thus far proven to be ideal. Therefore, more robust markers are still needed.
FXYD2, located on chromosome 11, is a single-pass type III membrane protein and the gamma subunit of trimeric Na+/K+-transporting ATPase whose regulatory function is to modulate the kinetic properties and stabilize the renal tubular Na+/K+-transporting ATPase.15, 16 Previous studies have shown that the FXYD2 protein is highly enriched in kidney tissue.17 Furthermore, it is expressed specifically in the distal nephron.
Previous studies indicate that renal oncocytomas and chromophobe RCCs are derived from the distal aspect of the nephron.9, 18, 19 In contrast, clear-cell and papillary RCCs are known to express proximal tubule markers. It is the aim of this study to explore the expression pattern of the distal tubule-specific protein, FXYD2, in RCCs and oncocytomas.
Materials and methods
Reagents
Commercial antibodies mouse anti-human Na+/K+-transporting ATPase gamma chain (sc-81876; Santa Cruz Biotechnology, San Diego, CA) and the secondary detection antibody goat anti-rabbit alkaline phosphatase (115-055-047) were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Synthetic peptides for immunization were prepared at Biomolecules Midwest (Waterloo, IL) and consisted of the target peptide, and an extended N-terminal long chain biotin and N-terminal cysteine version of this peptide. Full-length isoform 1 FXYD2 was synthesized and purified at AAPPTec (Louisville, KY). KLH-maleimide (1861615) was purchased from Pierce Chemical (Rockford, IL). Premade normal human kidney homogenate tissue blots (TB10) were from GBiosciences (Saint Louis, MO).
Antibody Production and Characterization
Two rabbits (9472 and 9473) were each immunized with an equal concentration mixture of KLH-P-4840 (G53GNKKRRQINEDE65) and KLH-P-4841 (G8GGSPKGDVDPFYY21) at Harlan Bioproducts for Sciences (Madison, WI). Individual rabbit anti-serum was affinity-purified over a SulfoLink P-4840 column with the non-bound flow-through applied to a SulfoLink P-4841 column. Bound anti-peptide specific antibody from each column was acid eluted, neutralized, and dialyzed at 4 °C against PBS, pH 7.2, containing 0.05% sodium azide as described.20 A direct binding assay with biotinylated peptide attached to plate-bound streptavidin was used to estimate the titer of the affinity-purified rabbit antibodies. In addition, another direct binding assay utilized plate-bound full-length synthetic FXYD2. Antibody concentration was determined by UV scanning from 240 to 320 nm, using A280=1.4 ml/(mg·cm) and then stored at 4 °C. The procedure for western blots has been described,21 and the alkaline phosphatase substrate for western blots 5-bromo,4-chloro,3-indoylphosphate/nitrobluetetrazolium was obtained from Kirkegaard and Perry (Gaithersburg, MD).
Immunohistochemistry
Single sections of formalin-fixed paraffin-embedded tissue were cut. Sections were stained using a Ventana Medical Systems Benchmark XT Autostainer. Following deparaffinization, antigen retrieval was performed using calcium citrate in 1 mM EDTA at pH 8.0. FXYD2 primary antibody (Rabbit polyclonal R-9472/P4841; 5 μg/ml final concentration) was applied and incubated at room temperature for 36 min. Other immunohistochemical staining procedures were performed according to the manufacturer’s instructions.
Fluorescence In Situ Hybridization
Interphase FISH was performed on formalin-fixed paraffin-embedded tissue sections cut at a thickness of 5 μm on positively charged microscope slides. The paraffin was removed from the sections with three washes of 5 min each in CitriSolve. The slides were then hydrated in two washes of absolute ethanol for 1 min each and allowed to air dry, after which the slides were processed through a pretreatment solution of sodium thiocyanate, which had been pre-heated to 80 °C. Following a 3-min wash in distilled water, the tissue was digested in protease solution (pepsin in 0.2 N HCl) for 15 min at 37 °C, followed by another 3 min wash in distilled water. The slides were allowed to air dry, after which they were dehydrated by passing through consecutive 70, 85, and 100% ethanol solutions for 1 min each, with air drying before applying the probe mixtures. Probes were centromeric-specific probes (CEP) for chromosomes 1, 2, 6, 10, 11, and 17, as well as a locus-specific (LSI) break-apart probe for MLL (11q23). All probes were from Abbott Molecular (Des Plaines, IL). For this study, CEP1 (SpectrumOrange) was paired with CEP10 (SpectrumGreen), and CEP6 (SpectrumAqua) was combined with CEP2 (SpectrumOrange) and CEP 17(SpectrumGreen). The dual-color MLL probe (SpectrumOrange/SpectrumGreen) was paired with CEP 11 (SpectrumAqua). In all cases, probes were diluted at 1:50 in CEP buffer (Abbott Molecular). Next, 10 μl of probe in buffer was applied to the appropriate slide and the section was coverslipped. Co-denaturation was achieved by incubating the slides at 73 °C for 5 min in a slide moat. Hybridization occurred by transferring the slides to a 37 °C light-shielded, humid slide moat overnight. Post hybridization, the cover slips were removed and the slides immersed in 75 °C wash solution (2 × standard saline citrate/0.3%NP40) for 2 min, followed by a 1-min wash in jar containing the same solution at room temperature. The slides were allowed to air dry in the dark and were then counterstained with 10 μl of DAPI II (Abbott Molecular). Slides were examined using an Olympus BX60 fluorescent microscope with appropriate filters for SpectrumOrange, SpectrumGreen, SpectrumAqua, and the DAPI counterstain. The signal patterns were documented using a CoolSnap camera and CytoVision Imaging System.
FISH Interpretation
A minimum of 200 neoplastic cells were counted for each tumor and for each probe type. A tumor was considered to have a monosomy (chromosomal loss) for a particular chromosome if the percentage of nuclei with only one signal for the probe was greater than 32, 33, 32, 29, or 33% for chromosomes 1, 2, 6, 10, and 17, respectively. Polysomy (chromosomal gain) was found for a particular chromosome if the percentage of nuclei with three or more signals for the probe was >10%, for chromosomes 1, 2, 6, 10, 11, and 17. The percentage of cells needed to consider a chromosomal loss or gain was established from a 95% confidence interval utilizing a reference range of normal cells.
Case Selection
The pathology records of Barnes-Jewish Hospital were searched for cases of renal oncocytoma and chromophobe RCC between 1998 and 2010. A total of 33 oncocytomas and 37 chromophobe RCCs were identified. Only cases with classical histology were included in the study. Cases with classical histology with conflicting Hale’s colloidal iron staining (one oncocytoma and one chromophobe RCC), FISH (one oncocytoma and four chromophobe RCC) results, and/or cytokeratin 7 staining (four oncocytoma and four chromophobe RCC) were excluded from the study for a total of 30 oncocytomas and 27 chromophobe RCCs. An additional 15 conventional clear-cell RCCs and 11 papillary RCCs were selected.
Results
Rabbit Anti-Peptide FXYD2
A tissue western blot is shown in Figure 1a for normal human kidney tissue homogenate immunoprobed with affinity-purified R-9472/P-4841. The prevalent positive western blot for the kidney tissue suggests that this antibody preparation may be useful for immunohistochemistry.
(a). Western blot of human kidney homogenate for FXYD2 using rabbit polyclonal antibody R-4972/P4841. A prominent band is seen at the expected molecular weight for FXYD2 (arrow). (b) Normal human kidney immunostained using rabbit anti-FXYD2. This low-power view shows the staining distribution predominantly in the corticomedullary junction ( × 20). (c). Higher-power view of normal human kidney immunostained using rabbit anti-FXYD2. The antibody highlights the basolateral aspect of the distal tubules.
Patient Characteristics
Of the 27 patients diagnosed with chromophobe RCC, 15 (56%) were women and 12 were men (44%). Their average age was 60±14 years. In contrast, patients diagnosed with renal oncocytoma had a higher proportion of men (21/30; 70%) than women (9/31; 30%). Patients with oncocytoma also tended to be older, average age 66±11 years, than patients with chromophobe RCC. There was no significant difference in the average size of chromophobe RCC, 5.2±3.5 cm, compared with oncocytoma, 3.7±2.1 cm. Although there is a trend toward oncocytomas presenting in older men compared with chromophobe RCC, the sample size is too small for meaningful statistical analysis.
Normal Kidney Tissue
Normal kidney tissue was stained with an affinity-purified rabbit anti-FXYD2 antibody R-9472 generated against an N-terminal peptide P-4841 (Figure 1). There was intense staining of the basolateral aspect of the distal convoluted tubules (Figures 1b and c). In contrast, the proximal tubules showed only faint cytoplasmic staining and lacked basolateral membrane reactivity (Figures 1b and c). Vascular collagen consistently demonstrated mild background staining. The glomeruli, interstitium, and vascular endothelium showed no appreciable staining. Normal kidney tissue stained with a commercial mouse monoclonal anti-FXYD2 antibody showed similar results (data not shown). There was no staining in the absence of primary antibody, 3,3′-diaminobenzidine substrate, or secondary antibody.
Renal Neoplasms
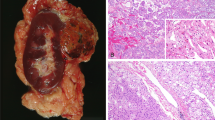
Single sections of chromophobe RCCs, renal oncocytomas, conventional clear-cell RCCs, and papillary RCCs were stained with a rabbit polyclonal anti-FXYD2 antibody (Figure 2). A subset of chromophobe RCCs and oncocytomas was evaluated using multiple ancillary techniques that have been reported to assist in discriminating renal oncocytoma from chromophobe RCC. Only cases with diffuse cytokeratin 7 staining, positive colloidal iron histochemistry, and monosomy for chromosomes 1, 2, 6, 10, and 17 were considered as chromophobe RCCs in this subset. If any case displayed discordant results using any of these tests, it was excluded from analysis. Similarly, cases histologically consistent with oncocytomas that showed diffuse cytokeratin 7 staining, colloidal iron staining, or monosomy for chromosomes 1, 2, 6, 10, and 17 were excluded from analysis. Of these tumors, 100% (7/7) of chromophobe RCCs were positive for FXYD2, whereas only 17% (2/12) of oncocytomas showed FXYD2 immunoreactivity. Importantly, 86% (6/7) of chromophobe RCCs stained with FXYD2 in a strong (≥2+) fashion. None of the oncocytomas showed more than weak (1+) patchy staining.
(a). Chromophobe RCC (H&E; × 100). (b) FXYD2 immunostain of chromophobe RCC from a demonstrates strong, diffuse membranous staining ( × 100). (c) Oncocytoma (H&E; × 100). (d) FXYD2 immunostain of oncocytoma from c shows no appreciable staining ( × 100). (e) Clear-cell RCC (H&E; × 100). (f) FXYD2 immunostain of the clear-cell RCC from E is negative ( × 100). (g) Papillary RCC (H&E; × 100). (h) FXYD2 immunostain of the papillary RCC from G is negative.
An additional 20 chromophobe RCCs and 18 oncocytomas were stained with FXYD2. The results are summarized in Table 1. FXYD2 was positive in 96% (26/27) of chromophobe RCCs in a distinctly membranous pattern. Three of these cases were eosinophilic variants of chromophobe RCC, of which three (100%) stained with FXYD2. In all, 93% (25/27) of the chromophobe RCCs showed ≥2+ staining intensity for FXYD2. In contrast, only 5 out of 30 (17%) oncocytomas showed positive immunostaining for FXYD2.
Chromophobe RCCs demonstrated variable FXYD2 staining patterns (Figure 3). Strong, diffuse staining, strong patchy staining, and weak diffuse staining were all observed (Figures 3a–c). Of the 26 chromophobe RCCs that were positive for FXYD2, 9 (35%) showed 3+ staining intensity, 16 (62%) showed 2+ intensity, and 1 (4%) showed 1+ intensity. Complete membranous staining was a constant feature. In the subset of oncocytomas that was FXYD2 positive, the staining was in a membranous pattern, but was in a patchy distribution and weak (1+) (Figure 3e). The sensitivity for discriminating chromophobe RCC from oncocytoma was 97% and specificity was 83%. FXYD2 showed immunoreactivity in 13% of clear-cell RCCs. No papillary RCCs showed FXYD2 immunoreactivity. FXYD2 immunohistochemistry was compared with kidney-specific cadherin (Ksp-cadherin) in the same subset of tumors that were evaluated using colloidal iron, FISH, and cytokeratin 7. One hundred percent (7/7) of chromophobe RCCs were stained with FXYD2 and Ksp-cadherin. However, 17% (2/12) and 33% (4/12) of oncocytomas were stained with FXYD2 and Ksp-cadherin, respectively (Table 2). The sensitivity for Ksp-cadherin in this series is 100% and specificity is 67%.
(a) Chromophobe RCC stained for FXYD2 shows a diffuse, strong staining pattern. (b) FXYD2 may result in a strong, patchy staining pattern as seen in this case of chromophobe RCC. (c) This example of chromophobe RCC shows diffuse but weak immunostaining for FXYD2. (d) Occasional negative FXYD2 staining may also be seen in chromophobe RCC. (e) Rare oncocytomas showed weak, patchy immunostaining for FXYD2. (f) Oncocytoma stained for FXYD2 shows no appreciable immunostaining. This was most commonly seen.
Discussion
Distinguishing chromophobe RCC from its benign mimic, renal oncocytoma, is a diagnostic challenge that continues to confront surgical pathologists. The eosinophilic variant of chromophobe RCC is particularly problematic because of its striking similarity to oncocytoma. Both of these tumor types are believed to derive from the distal nephron.9, 18, 19 Previous work has identified FXYD2, the gamma subunit of the Na+/K+-ATPase, as a distal tubule-specific protein that is highly enriched in human kidney tissue.17 The current study represents the first examination of FXYD2 in renal tumors.
Classical histomorphology, FISH, colloidal iron, and cytokeratin 7 immunohistochemical staining were applied as a gold standard in order to unequivocally characterize a subset of tumors as oncocytomas and chromophobe RCCs. Of this subset, 100% of chromophobe RCCs were positive for FXYD2 in a distinctly membranous pattern. Of these cases, 86% (6/7) showed strong (≥2+) membranous staining for FXYD2. In contrast, only 17% of oncocytomas showed FXYD2 immunostaining. Importantly, the two cases that were positive showed weak (1+) staining. When a larger group of tumors was stained for FXYD2, 97% of chromophobe RCCs were FXYD2 positive, 93% strongly (2+ or greater) positive, whereas 17% of oncocytomas showed only weak (1+) FXYD2 staining. FXYD2 immunohistochemistry showed a similar reaction pattern as Ksp-cadherin, albeit with a trend toward greater specificity for chromophobe RCC. However, the sample size used for comparing these two markers was too small for a robust statistical analysis.
There was variability in the staining pattern among chromophobe RCCs. This may be due to heterogenous expression of FXYD2 within individual tumors. A constant feature among chromophobe RCCs was circumferential membranous staining of tumor cells in either a diffuse or a patchy distribution. In contrast, the few oncocytomas that showed FXYD2 immunoreactivity did so in a weak and patchy distribution, with only rare cells demonstrating circumferential membranous staining. Further improvements in antibody-staining conditions may serve to alleviate some of this variability. Alternatively, these oncocytomas may represent hybrid tumors.22, 23
It is intriguing that the distal tubule-specific protein FXYD2 is preferentially expressed in chromophobe RCC when both chromophobe RCC and oncocytoma are thought to derive from the distal nephron. Previous investigations using mRNA-expression analysis demonstrated that chromophobe RCC shows increased expression of distal nephron genes when compared with oncocytoma.24 Preferential expression of FXYD2 in chromophobe RCC supports the hypothesis that renal oncocytoma and renal chromophobe RCC either differentiate from different cell types or differentiate along separate pathways, with chromophobe RCC retaining more distal nephron elements.24
The role, if any, of FXYD2 in the pathogenesis of chromophobe RCC is unclear. Previous studies have shown that FXYD2 is upregulated in cultured rat renal NRK-52E cells in response to hyperosmotic stress.25 Interestingly, upregulation of full-length FXYD2 had a negative effect on cell growth in cultured NRK-52E cells. Expression of a mutant FXYD2 resulting in loss of the C-terminus, however, was associated with loss of inhibition of cell growth in culture. Although speculative, these studies indicate a potential role for FXYD2 in modulating tumor cell growth. In the present study, an antibody to the N terminus was used for immunohistochemical analysis. Therefore, this antibody cannot detect a mutant, C-terminus truncated form of FXYD2.
There are several limitations to the current study. Although it is clear that FXYD2 preferentially reacts with chromophobe RCC, 17% of oncocytomas also showed weak FXYD2 staining. It is important to note that there are many cases of chromophobe RCC and oncocytoma where the diagnosis is clear based on histology alone. Thus, the potential utility of FXYD2 would be in ambiguous cases where other eosinophilic renal tumors are in the differential. We only had three eosinophilic variants of chromophobe RCC in our set, all of which stained with FXYD2. We did not do a rigorous evaluation of type 2 papillary RCCs or clear-cell RCCs with eosinophilic features. Because the eosinophilic variant of chromophobe RCC is more likely to be confused with oncocytoma, we analyzed these two tumors as a group. The positive predictive value of the FXYD2 test decreased from 84 to 38% because of the lower disease prevalence. The negative predictive value increased from 96 to 100%. This type of statistical analysis, however, is confounded by the low number of eosinophilic variants and the uncertainty of the true disease prevalence among cases that will actually be tested because not all oncocytomas will require immunohistochemical staining. Furthermore, if a cutoff of 2+ staining intensity is applied, then the FXYD2 test becomes 100% specific and 93% sensitive for chromophobe RCC. It is also unclear whether or not the oncocytomas with weak FXYD2 staining represented hybrid tumors. Future investigations using a group of well-characterized hybrid tumors and other mimics of chromophobe RCC are warranted. Our study is further limited by the fact that tumors were evaluated based on one representative section. Although tumor heterogeneity may have been missed because of this, the methods employed are representative of common surgical pathology practice. Despite these limitations, our results clearly demonstrate differential expression of FXYD2 in chromophobe RCC when compared with oncocytoma, conventional clear-cell RCC, and papillary RCC.
In conclusion, the present study is the first to demonstrate the expression profile of the Na+/K+-ATPase gamma subunit FXYD2 in renal tumors. The results indicate the potential utility of this biomarker in discriminating chromophobe RCC from other renal neoplasms, particularly renal oncocytoma. Further studies examining FXYD2 expression in a wide variety of renal tumors with eosinophilic features are warranted to examine the utility of this immunostain for routine pathology practice.
References
Ljungberg B, Campbell SC, Choi HY et al The epidemiology of renal cell carcinoma. Eur Urol 2011;60:615–621.
Cho E, Adami HO, Lindblad P . Epidemiology of renal cell cancer. Hematol Oncol Clin North Am 2011;25:651–665.
Paner GP, Amin MB, Alvarado-Cabrero I et al A novel tumor grading scheme for chromophobe renal cell carcinoma. Prognostic utility and comparison with Fuhrman nuclear grade. Am J Surg Pathol 2010;34:1233–1240.
Leibovich BC, Lohse CM, Crispen PL et al Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol 2010;183:1309–1315.
Capitanio U, Cloutier V, Zini L et al A critical assessment of the prognostic value of clear cell, papillary and chromophobe histological subtypes in renal cell carcinoma: a population-based study. BJU Int 2009;103:1496–1500.
Crotty TB, Farrow GM, Lieber MM . Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol 1995;154:964–967.
Ficarra V, Brunelli M, Cheng L et al Prognostic and therapeutic impact of the histopathologic definition of parenchymal epithelial renal tumors. Eur Urol 2010;58:655–668.
Kuehn A, Paner GP, Skinnider BF et al Expression analysis of kidney-specific cadherin in a wide spectrum of traditional and newly recognized renal epithelial neoplasms: diagnostic and histogenetic implications. Am J Surg Pathol 2007;31:1528–1533.
Truong LD, Shen SS . Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med 2011;135:92–109.
Speicher MR, Schoell B, Du Manoir S et al Specific loss of chromosomes 1, 2, 6, 10, 13, 17, and 21 in chromophobe renal cell carcinomas revealed by comparative genomic hybridization. Am J Pathol 1994;145:356–364.
Kovacs A, Kovacs G . Low chromosome number in chromophobe renal cell carcinoma. Genes Chromosomes Cancer 1992;4:267–268.
Thoenes W, Störkel S, Rupelt HJ . Histopathology and classification of renal cell tumors (adenomas, oncocytomas, carcinomas). The basic cytological and histopathological elements and their use for diagnostics. Pathol Res Pract 1986;181:125–143.
Leroy X, Moukassa D, Copin MC et al Utility of cytokeratin 7 for distinguishing chromophobe renal cell carcinoma from renal oncocytoma. Eur Urol 2000;37:484–487.
Thoenes W, Störkel S, Rumpelt HJ . Human chromophobe cell renal carcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol 1985;48:207–217.
Geering K . FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol 2006;290:F241–F250.
Arystarkhova E, Sweadner KJ . Splice variants of the gamma subunit (FXYD2) and their significance in regulation of the Na, K-ATPase in kidney. J Bioenerg Biomembr 2005;37:381–386.
Floyd RV, Wray S, Martin-Vasallo P et al Differential cellular expression of FXYD1 (phospholemman) and FXYD2 (gamma subunit of Na, K-ATPase) in normal human tissues: a study using high density human tissue microarrays. Ann Anat 2010;192:7–16.
Störkel S, Steart PV, Drenckhahn D et al The human chromophobe cell renal carcinoma: its probable relation to intercalated cells of the collecting duct. Virchows Arch B Cell Pathol Incl Mol Pathol 1989;56:237–245.
Osunkoya AO, Cohen C, Lawson D et al Claudin-7 and claudin-8: immunohistochemical markers for the differential diagnosis of chromophobe renal cell carcinoma and renal oncocytoma. Hum Pathol 2009;40:206–210.
Idol RA, Robledo S, Du HY et al Cells depleted for RPS19, a protein associated with Diamond Blackfan anemia, show defects in 18S ribosomal RNA synthesis and small ribosomal subunit production. Blood Cells Mol Dis 2007;39:35–43.
Laterza OF, Modur VR, Crimmins DL et al Identification of novel brain biomarkers. Clin Chem 2006;52:1713–1721.
Delongchamps NB, Galmiche L, Eiss D et al Hybrid tumor ‘oncocytoma-chromophobe renal cell carcinoma’ of the kidney: a repot of seven sporadic cases. BJU Int 2009;103:1381–1384.
Pavlovich CP, Walther MM, Eyler RA et al Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol 2002;26:1542–1552.
Rohan S, Tu JJ, Kao J et al Gene expression profiling separates chromophobe renal cell carcinoma from oncocytoma and identifies vesicular transport and cell junction proteins as differentially expressed genes. Clin Cancer Res 2006;12:6937–6945.
Sweadner KJ, Pascoa JL, Salazar CA et al Post-transcriptional control of Na,K-ATPase activity and cell growth by a splice variant of FXYD2 protein with modified mRNA. J Biol Chem 2011;286:18290–18300.
Acknowledgements
DLC was funded by Siemens Healthcare Diagnostics and the Department of Pathology and Immunology. We thank Diane Robirds and Julie Branson for their outstanding technical efforts for the FISH analysis and interpretation. We also thank the Anatomic and Molecular Pathology Core Lab, Department of Pathology and Immunology, Washington University School of Medicine for expert immunohistochemical staining.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gaut, J., Crimmins, D., Lockwood, C. et al. Expression of the Na+/K+-transporting ATPase gamma subunit FXYD2 in renal tumors. Mod Pathol 26, 716–724 (2013). https://doi.org/10.1038/modpathol.2012.202
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.202