Abstract
Prostate cancer represents a major contributor to cancer mortality, but the majority of men with prostate cancer will die of other causes. Thus, a challenge is identifying potentially lethal disease at diagnosis. Conflicting results have been reported when investigating the relationship between infiltration of lymphocytes and survival in prostate cancer. One of the mechanisms suggested is the recruitment of regulatory T cells (Tregs), a subpopulation of T cells that have a role in promoting tumor growth. Tregs counteract tumor rejection through suppressive functions on the anti-immune response but their prognostic significance is still unknown. We report here the results of a conducted case–control study nested in a cohort of men treated with transurethral resection of the prostate and diagnosed incidentally with prostate cancer. Cases are men who died of prostate cancer (n=261) and controls are men who survived >10 years after their diagnosis (n=474). Infiltration of both Thelper and Tcytotoxic cells was frequently observed and the majority of the Tregs were CD4+. Thelper or Tcytotoxic cells were not associated with lethal prostate cancer. However, we found a nearly twofold increased risk of lethal prostate cancer when comparing the highest with the lowest quartile of CD4+ Treg cells (95% confidence interval: 1.3–2.9). Our conclusion is that men with greater numbers of CD4+ Tregs in their prostate tumor environment have an increased risk of dying of prostate cancer. Identification of CD4+ Tregs in tumor tissue may predict clinically relevant disease at time of diagnosis independently of other clinical factors.
Similar content being viewed by others
Main
Prostate cancer is the most common cancer among European and American men and the second most common cancer diagnosed among men worldwide.1, 2 In the US, it represents the second greatest contributor to cancer mortality behind lung cancer, but the vast majority of men diagnosed with prostate cancer will die of other causes.2 Thus, a major challenge in the prostate cancer management is identifying potentially lethal disease at time of diagnosis. Gleason score, prostate specific antigen (PSA), and tumor extent are valuable for disease management but they cannot adequately distinguish indolent from aggressive disease. Additional prognostic markers are urgently needed to more effectively guide clinical decision making.
Decades ago, Burnet3 postulated about the ability of the immune system to detect tumor cells and eliminate them. The various subtypes of T lymphocytes have diverse roles and represent powerful components of this anti-tumor immune response. Several studies have demonstrated that CD8 cytotoxic T cells are capable of recognizing and destroying various tumor cells.4 Although the main function of CD4 helper T cells is to aid in maintaining the expansion of CD8 cytotoxic T cells, CD4 helper T cells are also capable of tumor cell eradication.5, 6
Despite the potential of inflammatory cells to protect against cancer development, chronic or recurrent inflammation has also been hypothesized to contribute to prostate cancer development, mainly owing to DNA damage caused by reactive molecules released by the inflammatory cells.7, 8 One indicator of previous cellular injury is focal atrophy. In the prostate gland, the presence of chronic inflammation is specifically associated with two subtypes of focal prostatic atrophy, post-atrophic hyperplasia, and simple atrophy.9, 10 These lesions, together referred to as proliferative inflammatory atrophy, have been suggested as regenerative lesions and precursors of prostatic adenocarcinoma, either directly or indirectly via progression to prostatic intraepithelial neoplasia.10
Conflicting results have been reported for the relationship between lymphocytic infiltration and survival in prostate cancer patients. Vesalainen et al11 reported that tumors with dense lymphocyte infiltration were associated with higher survival rates compared with those with fewer lymphocytes. On the other hand, greater numbers of CD4 helper T cells have been associated with poor disease outcomes including postoperative biochemical recurrence and cancer-specific death.12, 13, 14 These disparate results could potentially be explained by a more careful examination of the specific subtypes of T lymphocytes, some of which may allow the immune system to act as a promoter for the emergence of primary tumors. Recent studies that have focused on subsets of regulatory T lymphocytes (Treg), including CD4+ Treg cells and CD8+ Tregs, have demonstrated immune suppressive function either directly through cell–cell contact or indirectly through the secretion of anti-inflammatory cytokines, such as TGF-β (transforming growth factor beta) and IL-10.15, 16 A recent paper by Miller et al17 revealed an increased prevalence of Tregs in prostate cancer tissue compared with normal prostate tissue from the same patient. Although follow-up time was limited, there was also the suggestion of an association between Treg cells and survival among men with prostate cancer.15 Significant differences in the Treg cell prevalence between the early and advanced gastric cancer and esophageal cancer have also been reported. In both diseases, patients with high proportion of Treg cells showed poorer survival rates compared with those with low proportion.18
The majority of previous studies have utilized CD25 as a biomarker for Treg cells. However, the most specific marker presently to identify Tregs is FOXP3, a member of the forkhead box family of transcription factors.19 In this population-based nested case–control study of men diagnosed with localized prostate cancer and extensive follow-up time (up to 30 years) after diagnosis, we have evaluated the role of CD4 helper T (Thelper) cells, CD8 cytotoxic T (Tcytotoxic) cells, CD4+FOXP3+ regulatory T (CD4+Treg) cells, and CD8+FOXP3+ regulatory T (CD8+Treg) cells in tumor tissue with respect to acute or chronic inflammation, type of atrophy, prostatic intraepithelial neoplasia, and lethal prostate cancer.
Materials and methods
Tissue Collection and Preparation
The present study is nested within a cohort of men with localized prostate cancer diagnosed in the Örebro and South East Health Care Regions of Sweden between 1977 and 1999, as described in detail previously.20, 21, 22 We initially identified a cohort of 1367 men during the study period. Eligible patients were identified through the population-based prostate cancer quality database in these regions. We included men who were diagnosed with incidental prostate cancer through transurethral resection of the prostate or adenoma enucleation, ie, category T1a-b tumors. In accordance with standard treatment protocols, patients with early-stage/localized prostate cancer were followed expectantly (‘watchful waiting’).
The study cohort was followed for cancer-specific and all-cause mortality until 1 March 2006, through record linkages to the Swedish Death Register and Migration Register. We obtained information on cause of death for each individual through a complete review of medical records by a study end points committee. Deaths were classified as cancer specific when prostate cancer was the primary cause of death.
Because visual histopathological evaluations and immunohistochemistry (IHC) for archival specimens is both time consuming and expensive, we utilized a novel nested study design that included men who either died from prostate cancer during follow-up (lethal prostate cancer ‘cases’, n=261) or who survived at least 10 years following their diagnosis (indolent prostate cancer ‘controls’, n=474). The study design excluded men with non-informative outcomes who either died from other causes within 10 years after cancer diagnosis, or did not die of prostate cancer and did not have the opportunity to survive 10 years before the end of study follow-up in 2006. We also excluded cases without complete data for both IHC and inflammation/atrophy variables.
Tumor areas marked on H&E slides for the corresponding paraffin-embedded formalin-fixed blocks were re-reviewed by a single pathologist (MF) blinded to disease outcome and other clinical data to confirm cancer status, Gleason score and other notable histopathological features. The Gleason scoring was performed according to the 2005 ISUP recommendations.23 All slides were assessed for the presence and type of inflammation, either acute or chronic, according to cells of the inflammatory infiltrate. Chronic inflammation was semi-quantitatively graded as mild, moderate or severe when the area of non-neoplastic prostate tissue covered by inflammatory cells was ≤10, ≥10–20, and ≥20%, respectively. Focal prostate atrophy was characterized according to the atrophy classification, proposed in 2006 by the Working Group for Histologic Classification of Prostate Atrophy Lesions with the following subtypes: simple atrophy, simple atrophy with cyst formation, post-atrophic hyperplasia, and partial atrophy. Given that simple atrophy with cyst formation and partial atrophy were rare (6 and 2%, respectively), we did not evaluate these lesions in the present study. A previous publication describes the association between the histopathologically assessed inflammation, atrophy, and prostatic intraepithelial neoplasia variables with lethal prostate cancer.24
Immunohistochemistry
Tissue cores with a diameter of 0.6 mm were collected from each transurethral resection of the prostate specimen. Three cores were taken from each patient to address potential tumor heterogeneity. Tissue microarrays were constructed with a Beecher manual arrayer and tissue microarray sections (4 μm) were used for IHC. Deparaffination, rehydration, and antigen retrieval was performed using Target Retrieval Solution (DAKO, Denmark) at pH 9.0. Primary antibodies used for the single staining were mouse monoclonal ready to use antibodies against CD4 (clone 4B12, DAKO), CD8 (clone C8/144, DAKO). Single IHC staining was performed with DAKO autostainer LINK system. The slides were incubated with primary antibodies at room temperature for 20 min and detected with EnVisionTM FLEX+, High pH, (Link) (DAKO). For the triple staining, we used as primary antibodies a mouse monoclonal and rabbit monoclonal ready to use multiplex cocktail against CD4 and CD8 (clone BC/1F6+SP16, Biocare Medical, Concord, USA) and mouse monoclonal antibody against FOXP3 (clone 236A/E7, eBioscience, San Diego, USA) at 1:100 dilution. After primary antibody incubation for 30 min at room temperature, slides were treated with secondary antibodies and chromogen for detection. To identify CD4 and CD8, Mach 2 doublestain 2 (Biocare Medical) and diaminobenzidin (Biocare Medical) and Warp Red Chromogen kit (Biocare Medical) were used, respectively. To detect FOXP3, Mach 2 mouse HRP-Polymer (Biocare Medical) served as secondary antibody followed by Vina Green Chromogen Kit (Biocare Medical) for visualization. Slides were then counterstained with hematoxylin. Tonsil was used as positive control.
Evaluation of CD4, CD8, and Foxp3 Expression
We quantified Thelper cells by CD4 protein expression, Tcytotoxic cells by CD8 expression, CD4+Treg cells by simultaneous CD4 and FOXP3 expression, and CD8+Treg cells by simultaneous CD8 and FOXP3 expression using a light microscope at × 40 magnification. All Treg cells were counted. For Thelper and Tcytotoxic cells, up to 50 cells were counted; greater numbers of positive cells were recorded in a single category as >50. The observers (SD and A-LO) were blinded to all the clinical data and conducted evaluations independently.
Statistical Analyses
To evaluate the association between clinical covariates and lethal case vs indolent control status, we used χ2-tests for categorical variables and t-tests for continuous variables. We used unconditional logistic regression to estimate odds ratios and 95% confidence intervals for the association between the mean number of Thelper lymphocytes, Tcytotoxic lymphocytes, and CD4+Treg cells across an individual patient’s three tissue cores with respect to the following outcomes: (1) presence of the histopathological characteristics as assessed visually by the study pathologist (MF); and (2) lethal prostate cancer. The histopathological characteristics included acute inflammation, chronic inflammation (none/mild vs moderate/severe), simple atrophy, post-atrophic hyperplasia, and prostatic intraepithelial neoplasia. Statistical significance was determined by a Wald test for the continuous cell count variables. Mixed models were used to assess the association beween Thelper, Tcytotoxic, and Treg cells at the core level. To aid in the interpretation of the odds ratios for lethal prostate cancer, we also estimated odds ratios according to quartiles of the T-cell subtype variables. In addition to univariate analyses, we also ran models adjusted for tumor stage, tumor percent, and primary and secondary Gleason grade. We hypothesized that visually assessed inflammation and atrophy/prostatic intraepithelial neoplasia lesion variables could act synergistically with the specific IHC-assessed T-cell subtypes to predict prostate cancer progression. Thus, we assessed the potential interactions between histopathologically assessed variables and number of Thelper, Tcytotoxic, and Treg cells with respect to lethal prostate cancer. To assess statistical significance of the interactions, we used a Wald χ2-test to compare unconditional logistic regression models that main effects and the product term of the mean number of positive cells and the dichotomous variable for lesion type, to models without the product term. All statistical analyses were carried out using SAS Statistical Software version 9.2 (Cary, NC). The study was approved by the Ethical Review Boards in Örebro and Linköping, Sweden.
Results
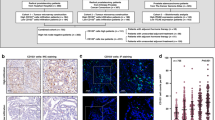
The clinical characteristics of the lethal cases and indolent controls are presented in Table 1. When IHC was used for visualization, FOXP3 expression was localized in the nuclei of the T cells, whereas CD4 and CD8 expression was localized in the cell membrane. The lymphocytes were predominantly located in the stroma surrounding the tumor, either as single cells or as aggregates. Intraepithelial immune cells were also seen but only as scattered cells (Figures 1a and b). The majority of the FOXP3+ cells were also CD4+ (Figures 1c and d). The mean counts of Thelper cells, Tcytotoxic cells, and CD4+Treg cells in an individual patient were 18, 27, and 2, respectively. Only three patients had CD8+ Treg cells; these cells were not evaluated further.
CD4 (brown), CD8 (red), and FOXP3 (green) expression in prostate tissue. (a) (magnification × 60) Immune cells located in the stroma as a aggregate. (b) (magnification × 100) Immune cells located in the epithelia and in the stroma. (c) (magnification × 60) Arrows indicate CD4+FOXP3+ T cells. (d) (magnification × 100). Solid arrow indicate a CD4+FOXP3+ T cell and open arrow indicate a CD8+FOXP3+ T cell.
Simultaneous infiltration of both Thelper and Tcytotoxic cells was frequently observed. Thelper cells and CD4+ Treg cells were positively moderately correlated within an individual patient (r=0.38) and within a single tissue core (β estimate=0.08, P<0.0001). Similarly, a positive correlation was also seen between Tcytotoxic cells and CD4+Tregs at both the patient and tissue core levels (r=0.26; and β estimate=0.06, P<0.0001). Thelper, Tcytotoxic, and CD4+ Treg cells were not associated with Gleason score or tumor stage. However, percent of tumor in the specimen was significantly inversely correlated with number of Thelper cells (r=−0.11; P=0.004) and Tcytotoxic cells (r=−0.14; P=0.0002).
We assessed the relationship between the various lymphocytes and the presence of inflammation, atrophy, and prostatic intraepithelial neoplasia lesions apparent by visual histopathological evaluation (Table 2). The mean numbers of all three T-cell subtypes were significantly and positively associated with the presence of moderate/severe chronic inflammation as assessed semi-quantitatively by the study pathologist, with the strongest association being apparent for CD4+ Tregs (odds ratio: 1.07; 95% confidence interval: 1.02–1.13). We also observed significant associations with acute inflammation for both the mean number of Thelper cells (odds ratio: 1.02; 95% confidence interval: 1.00–1.04) and the mean number of CD4+ Tregs (odds ratio: 1.06; 95% confidence interval: 1.01–1.12). We found that the association between the mean number of CD4+ Treg cells was statistically significantly and inversely associated with the odds of having simple atrophy visible in the tumor specimen (odds ratio: 0.94; 95% confidence interval: 0.89–0.99). The mean number of Tcytotoxic cells was inversely associated with the presence of prostatic intraepithelial neoplasia (odds ratio: 0.98; 95% confidence interval: 0.96–0.99).
We investigated whether Thelper cells, Tcytotoxic cells, or CD4+ Treg cells were associated with a lethal prostate cancer (Table 3). Using the mean cell count value across the three cores, we observed no association between the infiltration of Thelper cells or Tcytotoxic cells in the univariate or multivariate analyses. By contrast, every additional CD4+ Treg cell was associated with a 12% increase in odds of dying of prostate cancer (odds ratio:1.12; 95% confidence interval: 1.02–1.23) after adjustment for tumor stage, tumor percent, and primary and secondary Gleason score. When the mean number of cell counts across cores was categorized into quartiles, we observed a nearly twofold increase in the odds of dying of prostate cancer when comparing the highest with the lowest quartile of CD4+ Treg cells (odds ratio: 1.98; 95% confidence interval: 1.15–3.40).
We also explored interactions between acute and chronic inflammation, simple atrophy, post-atrophic hyperplasia and mean number of Thelper, Tcytotoxic, and CD4+ Treg cells (data not shown). The association between Thelper cells and lethal prostate cancer was modified by the presence of post-atrophic hyperplasia in the multivariate model (P-interaction=0.05). Among men without post-atrophic hyperplasia present, an increase in Thelper cells was not significantly associated with the odds of dying of prostate cancer (odds ratio: 1.00; 95% confidence interval: 0.98–1.02). Among men with post-atrophic hyperplasia, every one-unit increase in Thelper cells was associated with a 5% increase in the odds of lethal prostate cancer (odds ratio: 1.05; 95% confidence interval: 1.01–1.09). No other statistically significant interactions between lymphocyte types or between the lymphocytes and inflammation/atrophy/prostatic intraepithelial neoplasia were observed.
Discussion
Our data suggest at least two distinct pathways leading to lethal prostate cancer: (1) post-atrophic hyperplasia combined with evidence of moderate/severe chronic inflammation and a shift in the CD4/CD8 T-cell ratio; and (2) inflammation-independent, tumor-assisted increase of the CD4+ Treg population. These findings may explain why previous studies of lymphocytic infiltration that did not characterize specific T-lymphocyte subtypes produced apparently inconsistent associations with prostate cancer outcomes.11, 12, 13
In a previous study, in the same study population, chronic inflammation in the presence of post-atrophic hyperplasia was associated with greater likelihood of prostate cancer death.24 The results obtained in the present study shows that the Thelper cells, specifically interact with post-atrophic hyperplasia to influence cancer progression. Among men with post-atrophic hyperplasia, every additional CD4 helper T cell increases the risk of dying of prostate cancer. It has been suggested that inactivity of a substantial proportion of T-lymphocytes found in carcinoma leads to an ineffective anti-tumor immune response.25 Our data do not address the function of infiltrating CD4 helper T cells or the impact they may have on the prostate tissue as this will require a more thorough phenotypic analysis of the lymphocytes.
Accumulating evidence suggests that Treg cells are recruited to a variety of human carcinomas where they suppress the anti-tumor immune response performed mainly by Thelper and Tcytotoxic lymphocytes, and that infiltration may predict poor survival.18 Although neither infiltration of total CD4 helper T cells nor CD8 cytotoxic T cells was associated with lethal prostate cancer, a more detailed evaluation of the T-cell subpopulations in the tumor environment revealed that it is in fact the number of CD4+ Tregs cells that predict worse outcome in prostate cancer. We found that every additional CD4+ Treg cell was associated with a statistically significant 12% increase in prostate cancer death independently of other clinical factors. We also found a moderate positive correlation between the presence of CD4+ Treg cells and both Thelper cells and Tcytotoxic cells, indicating that the T cell populations involved in tumor eradication may be more important than the total burden of T cells.
Our data support the hypothesis that prostate cancer tumors recruit Tregs that suppress antitumor immunity and thereby aid tumor growth. A study of 20 prostate cancer patients undergoing prostatectomy for localized prostate adenocarcinoma by Sfanos et al26 found enrichment of Treg cells in almost all cases. Fox et al.15 identified FOXP3-positive cells in 126 of 146 tissue specimens obtained from prostate cancer patients. They were, however, unable to demonstrate a correlation between numbers of Tregs and PSA rise, as an indicator of survival, due to a limited follow-up time. Other recent studies show an enhanced frequency of Treg cells with a suppressive function in lymphocytes obtained both from prostate tissue and peripheral blood of patients with prostate cancer.16, 27 In addition, Sotosek et al28 found statistically significant higher levels of Treg cells in peripheral blood lymphocytes of prostate cancer patients when compared with benign prostatic hyperplasia patients and healthy volunteers. Further they suggest, in line with our study, that upregulation of Treg cells could be essential for tumor progression, as a positive correlation between PSA values and frequency of Treg cells was detected.
There are several possible explanations for the increased infiltration of Treg cells in the tumor area. First, tumor cells or macrophages inside the tumor are able to secrete the chemokine CCL22, which has affinity for the receptor CCR4, expressed on Treg cells.29 Second, cytokines secreted by the prostate tumors, such as TGF-β, can upregulate FOXP3 expression and expand the Treg population.30 TGF-β is a multi-functional cytokine that increases survival and proliferation of transformed cells, including transformed prostate epithelial cells, and it has been found in elevated levels in patients with metastatic disease.31
To our knowledge, it is the first study to investigate the role of Treg cells with respect to prostate cancer mortality. We assessed mortality from prostate cancer using >30 years of follow-up time after diagnosis among a cohort of men diagnosed with localized prostate cancer. We quantified the Tregs based on FOXP3 staining, as FOXP3 currently is considered to be the best single marker of Treg using a monoclonal antibody (236A/E7) found to be the most specific clone and recommended for IHC on paraffin-embedded tissue.32 To further strengthen the evaluation, we quantified FOXP3-positive cells based on a triple staining (CD4, CD8, and FOXP3) following recent studies that identified FOXP3 expression in prostate tumor cells.33 However, all prostate cancer patients included in the present study were diagnosed by transurethral resection of the prostate or adenoma enucleation before the PSA era. Given that PSA testing and needle biopsies are more often utilized for prostate cancer diagnosis in contemporary practice, further studies in PSA screened population and biopsy specimens will be required to verify the generalizability of our findings.
Chronic inflammation is common in tumor specimens from prostatectomy, transurethral resection of the prostate, and biopsy samples.7, 10, 24 In line with previous reports,12, 14, 34 our study revealed a high degree of infiltration of both Thelper cells as determined by CD4 expression and Tcytotoxic cells as determined by CD8 expression. The present study also demonstrates that IHC markers of inflammation are positively related to histopathological classification of inflammation, as all of the three T-cell subtypes were positively associated with the presence of moderate/severe chronic inflammation. In future studies, it will also be important to consider the potential sources of the common occurrence of prostatic inflammation. Bacteria are well-known to trigger infection and subsequently inflammatory response. Recent reports have identified Propionibacterium acnes as the most frequently observed bacterium in prostate tissue from men with prostate cancer.35, 36 The results obtained in the present study support the importance of continued investigation regarding the role of infectious agents and other modifiable contributors of prostatic inflammation in prostate cancer development and progression.
In summary, our data provide evidence that men with greater numbers of CD4+ Tregs in their prostate tumor environment have an increased risk of dying of their disease. In addition, the outcome of the disease in men with evidence of post-atrophic hyperplasia varies according to the number of CD4 helper T cells present. Our findings suggest that identification of CD4+ Tregs in tumor tissue predicts clinically relevant prostate cancer at time of diagnosis independently of other clinical factors.
References
Black RJ, Bray F, Ferlay J et al. Cancer incidence and mortality in the European Union: cancer registry data and estimates of national incidence for 1990. Eur J Cancer 1997;33:1075–1107.
Jemal A, Siegel R, Xu J et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300.
Burnet FM . The concept of immunological surveillance. Prog Exp Tumor Res 1970;13:1–27.
Nguyen T, Naziruddin B, Dintzis S et al. Recognition of breast cancer-associated peptides by tumor-reactive, HLA-class I restricted allogeneic cytotoxic T lymphocytes. Int J Cancer 1999;81:607–615.
Appay V, Zaunders JJ, Papagno L et al. Characterization of CD4(+) CTLs ex vivo. J Immunol 2002;168:5954–5958.
Carlos TM . Leukocyte recruitment at sites of tumor: dissonant orchestration. J Leukoc Biol 2001;70:171–184.
Blumenfeld W, Tucci S, Narayan P . Incidental lymphocytic prostatitis. Selective involvement with nonmalignant glands. Am J Surg Pathol 1992;16:975–981.
Nelson WG, De Marzo AM, Isaacs WB . Prostate cancer. N Engl J Med 2003;349:366–381.
Aus G, Abbou CC, Bolla M et al. EAU guidelines on prostate cancer. Eur Urol 2005;48:546–551.
De Marzo AM, Marchi VL, Epstein JI et al. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol 1999;155:1985–1992.
Vesalainen S, Lipponen P, Talja M et al. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer 1994;30A:1797–1803.
Irani J, Goujon JM, Ragni E et al. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Pathologist Multi Center Study Group. Urology 1999;54:467–472.
Karja V, Aaltomaa S, Lipponen P et al. Tumour-infiltrating lymphocytes: a prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res 2005;25:4435–4438.
McArdle PA, Canna K, McMillan DC et al. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br J Cancer 2004;91:541–543.
Fox SB, Launchbury R, Bates GJ et al. The number of regulatory T cells in prostate cancer is associated with the androgen receptor and hypoxia-inducible factor (HIF)-2alpha but not HIF-1alpha. Prostate 2007;67:623–629.
Kiniwa Y, Miyahara Y, Wang HY et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res 2007;13:6947–6958.
Miller AM, Lundberg K, Ozenci V et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol 2006;177:7398–7405.
Kono K, Kawaida H, Takahashi A et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother 2006;55:1064–1071.
Hori S, Nomura T, Sakaguchi S . Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–1061.
Andren O, Fall K, Franzen L et al. How well does the Gleason score predict prostate cancer death? A 20-year followup of a population based cohort in Sweden. J Urol 2006;17:1337–1340.
Aus G, Robinson D, Rosell J et al. Survival in prostate carcinoma—outcomes from a prospective, population-based cohort of 8887 men with up to 15 years of follow-up: results from three countries in the population-based National Prostate Cancer Registry of Sweden. Cancer 2005;103:943–951.
Johansson JE, Andren O, Andersson SO et al. Natural history of early, localized prostate cancer. JAMA 2004;291:2713–2719.
Montironi R, Cheng L, Lopez-Beltran A et al. Original Gleason system versus 2005 ISUP modified Gleason system: the importance of indicating which system is used in the patient’s pathology and clinical reports. Eur Urol 2010;58:369–373.
Davidsson S, Fiorentino M, Andren O et al. Inflammation, focal atrophic lesions, and prostatic intraepithelial neoplasia with respect to risk of lethal prostate cancer. Cancer Epidemiol Biomarkers Prev 2011;20:2280–2287.
Nishikawa H, Jager E, Ritter G et al. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood 2005;106:1008–1011.
Sfanos KS, Bruno TC, Maris CH et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res 2008;14:3254–3261.
Yokokawa J, Cereda V, Remondo C et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res 2008;14:1032–1040.
Sotosek S, Sotosek Tokmadzic V, Mrakovcic-Sutic I et al. Comparative study of frequency of different lymphocytes subpopulation in peripheral blood of patients with prostate cancer and benign prostatic hyperplasia. Wien Klin Wochenschr 2011;123:718–725.
Curiel TJ, Coukos G, Zou L et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942–949.
Barrack ER . TGF beta in prostate cancer: a growth inhibitor that can enhance tumorigenicity. Prostate 1997;31:61–70.
Jakowlew SB . Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev 2006;25:435–457.
Roncador G, Brown PJ, Maestre L et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol 2005;35:1681–1691.
Ebert LM, Tan BS, Browning J et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res 2008;68:3001–3009.
Valdman A, Jaraj SJ, Comperat E et al. Distribution of Foxp3-, CD4- and CD8-positive lymphocytic cells in benign and malignant prostate tissue. APMIS 2010;118:360–365.
Cohen RJ, Shannon BA, McNeal JE et al. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J Urol 2005;173:1969–1974.
Fassi Fehri L, Mak TN, Laube B et al. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. Int J Med Microbiol 2010;301:69–78.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Davidsson, S., Ohlson, AL., Andersson, SO. et al. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3+ regulatory T cells with respect to lethal prostate cancer. Mod Pathol 26, 448–455 (2013). https://doi.org/10.1038/modpathol.2012.164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.164
Keywords
This article is cited by
-
The gut microbiome-prostate cancer crosstalk is modulated by dietary polyunsaturated long-chain fatty acids
Nature Communications (2024)
-
Engineering prostate cancer in vitro: what does it take?
Oncogene (2023)
-
Bee gomogenat rescues lymphoid organs from degeneration by regulating the crosstalk between apoptosis and autophagy in streptozotocin-induced diabetic mice
Environmental Science and Pollution Research (2022)
-
Neoadjuvant rituximab modulates the tumor immune environment in patients with high risk prostate cancer
Journal of Translational Medicine (2020)
-
T cells CD4+/CD8+ local immune modulation by prostate cancer hemi-cryoablation
World Journal of Urology (2020)