Abstract
Except for the well-known immunoglobulin G (IgG) producing cell types, ie, mature B lymphocytes and plasma cells, various non-lymphoid cell types, including human cancer cells, neurons, and some specified epithelial cells, have been found to express IgG. In this study, we detected the expression of the heavy chain of IgG (IgGγ) and kappa light chain (Igκ) in papillary thyroid cancer cells. Using in situ hybridization, we detected the constant region of human IgG1 (IGHG1) in papillary thyroid cancer cells. With laser capture microdissection followed by RT-PCR, mRNA transcripts of IGHG1, Igκ, recombination activating gene 1 (RAG1), RAG2, and activation-induced cytidine deaminase genes were successfully amplified from isolated papillary thyroid cancer cells. We further confirmed IgG protein expression with immunohistochemistry and found that none of the IgG receptors was expressed in papillary thyroid cancer. Differences in the level of IgGγ expression between tumor size, between papillary thyroid cancer and normal thyroid tissue, as well as between papillary thyroid cancer with and without lymph node metastasis were significant. Taken together, these results indicate that IgG is produced by papillary thyroid cancer cells and that it might be positively related to the growth and metastasis of papillary thyroid cancer cells. Furthermore, it was demonstrated that IgGγ colocalized with complement proteins in the same cancer cells, which could indicate that immune complexes were formed. Such immune complexes might consist of IgG synthesized by the host against tumor surface antigens and locally produced anti-idiotypic IgG with specificity for the variable region of these ‘primary’ antibodies. The cancer cells might thus escape the host tumor-antigen-specific immune responses, hence promoting tumor progression.
Similar content being viewed by others
Main
Immunoglobulin G (IgG) is an important component of the adaptive immune system and constitutes 75–80% of total Ig.1 In recent years, in addition to mature B cells and plasma cells, other cell types with the capacity to produce IgG have been identified. Such cell types include neurons, mammary gland epithelial cells, eyes, endothelial cells of the umbilical cord, and trophoblasts (J Gu, unpublished data).2, 3, 4, 5 IgG expression has also been found in colon carcinoma, breast carcinoma, lung carcinoma, and soft tissue tumors.6, 7, 8, 9 The exact function of IgG in cancers has not yet been elucidated, but IgG might be involved in the promotion of tumor growth based on preliminary in vitro evidence of its capacity to stimulate cell proliferation and invasion.6, 10 Another observation supporting a growth-promoting role of IgG include the observed correlation between the level of IgG expression and tumor grade and stage in soft tissue tumors.9 To date, it is not known whether papillary thyroid cancer cells also produce IgG. Lucas et al,11 detected cellular deposits of IgG in 80% of 41 papillary thyroid cancer cases studied. They, however, assumed that the presence of IgG was the result of deposition from the serum, and not of local production.11
Papillary thyroid cancer is the most common endocrine malignancy.12 It is well differentiated and is one of the most curable cancers.13 However, there are also factors associated with a less favorable outcome including age (>45 years), large tumor size (>5 cm), histology (tall cell variant histology), extrathyroidal extension, distant metastases, lymph nodal metastases, and positive family history.14, 15, 16, 17
Another important component of the body's immune defense arsenal that has been identified in papillary thyroid cancer tissues is the complement system. The complement system is composed of several serum proteins that assist in the elimination of invading pathogens and modified self-cells.18 The complement cascade can be initiated via three major pathways, namely, the classical, alternative, and lectin pathway.18 The liver is the main production site, but other locations include the renal epithelial cells, the small intestines, and the brain.19 Complement protein mRNA synthesis has been demonstrated in colon adenomas and carcinomas.20 In papillary thyroid cancer, C3d, C4d, C5, and C5b-C9 have been detected at the protein level, but it is currently unknown whether complement proteins are also produced by papillary thyroid cancer cells.11, 21
In cancer immunology, complement proteins are thought to contribute to antitumor immune surveillance.22, 23 Mechanisms that might be involved include complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, direct inhibition of tumor growth, and mobilization of hematopoietic progenitor cells.22, 23 Lucas et al11 suggested that the co-expression of IgG and C1q indicated a tumor-specific host immune response with initiation of the classical complement pathway through binding of IgG to tumor antigens. Yamakawa et al21 demonstrated the expression of membrane complement regulatory proteins (mCRPs), including membrane cofactor protein (CD46), decay-accelerating factor (CD55), and protectin (CD59), and suggested that these factors protect papillary thyroid cancer cells from cell lysis induced by the activation of the complement system.
Here, we studied the expression of IgGγ and Igκ in papillary thyroid cancer using immunohistochemistry, in situ hybridization, and laser capture microdissection-assisted RT-PCR. Using a papillary thyroid cancer tissue microarray, we also investigated the correlation between IgG expression and various clinicopathological features. In addition, expression of C1q, C3c, and C4c were examined by immunohistochemistry. In situ hybridization with a probe directed against C3 was performed to study whether local C3 production occurs in papillary thyroid cancer. In order to exclude the possibility that IgG had been transported across the cancer cell membrane into the cell by means of Fc receptors, we also studied the expression of IgG-specific receptors, ie, the three FcγR subtypes (FcγRI/CD64, FcγRII/CD32, and FcγRIII/CD16) and the neonatal Fc receptor (FcRn).24, 25 In line with our expectations, papillary thyroid cancer cells were indeed found to produce IgG, as evidenced by the detection of both proteins and mRNA transcripts. IgG expression correlated well with large tumor size and presence of local lymph node metastases, but not with other clinicopathological features. In addition, C1q, C3c, and C4c were detected in papillary thyroid cancer cells. However, only the proteins, but not the mRNA transcripts of C3, were identified in papillary thyroid cancer cells. Furthermore, co-expression of IgG and C1q suggested activation of the classical pathway through the formation of immune complexes. Unlike Lucas et al,11 who proposed that such immune complexes consist of IgG binding to papillary thyroid cancer surface antigens, we suggest that they are composed of IgG synthesized by the host against papillary thyroid cancer surface antigens and anti-idiotypic IgG produced by the cancer cells with specificity for the variable region of these host antibodies. Formation of immune complexes prevents host IgG from binding to its antigen, thus protecting the cancer cells from antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity, resulting in promotion of tumor growth, progression, and metastasis.
Materials and methods
Tissues
Fresh tissues were obtained by thyroidectomy from 26 patients with papillary thyroid cancer, and formalin-fixed, paraffin-embedded biopsy specimens were obtained from 18 patients suffering from papillary thyroid cancer. Four specimens were follicular-variant papillary thyroid cancer subtype. Others were of the usual papillary thyroid cancer subtype. In total, there were 44 patients, 40 females (90%), mean age: 45 years, age range: 43±12 years, 15 with presence of lymph node metastasis (34%). All tissues were collected from the Pathology Department of Shantou University Medical College. The fresh tissues were immediately fixed in 4% paraformaldehyde, dehydrated in increasing ethanol concentrations and embedded in paraffin. The remaining fresh tissues were quickly frozen in nitrogen and stored at −70°C. A human papillary thyroid cancer tissue microarray was obtained from Alenabio (Xi’an, China). All tissues on the tissue array were usual PTC. The formalin-fixed, paraffin-embedded tissue microarray contained 55 tissue samples comprising 45 papillary thyroid cancer tissues and 10 normal thyroid tissues. The clinicopathological features of the papillary thyroid cancer cases displayed on the tissue microarray are summarized in Table 3. Informed consent was obtained from all patients, and the study was performed in accordance with the Declaration of Helsinki Principles.
Immunohistochemistry
Serial sections (4 μm thick) were deparaffinized and hydrated in decreasing concentrations of ethanol. The slides were incubated overnight with the following primary antibodies: IgGγ, Igκ, C1q, C3c, C4c, CD16, CD32, CD64, and FcRn. Cytokeratin (CK) was used as a marker of papillary thyroid cancer cells, and CD20 was used to exclude the contamination by B cells. The details of primary antibodies are shown in Table 1. PBS was used as a negative control. We used human spleen tissue as the positive control of antibodies Igγ, Igκ, CD16, CD32, CD64, and FcRn. Human liver tissue was used as the positive control of C1q, C3c, C4c, and CK. After incubation with appropriate secondary antibodies (PV9000 or PV9003, Zymed Laboratory, South San Francisco, USA) and washing, the slides were visualized with 3-amino-9-ethyl-carbazole (AEC; Golden Bridge International, New York, USA) and counterstained with hematoxylin. Immunohistochemistry was performed on both the paraformaldehyde-fixed samples and the microarray.
Generation of Digoxigenin-Labeled cRNA Probes
The generation of digoxigenin-labeled human IGHG1 cRNA probe was performed as previously described.7
Probes specific for the human C3 were prepared from fresh human liver tissue. Total RNA was extracted by Trizol reagent (Invitrogen, California, USA), and reverse transcription was performed using random primers and first strand cDNA synthesis kit (Toyobo, Osaka, Japan) following manufacturer's instructions. PCR primers for human C3 gene were 5′-TCGGATGACAAGGTCACCCT-3′ (sense), 5′-GACAACCATGCTCTCGGTGAA-3′ (antisense).20 The 407-bp size product was extracted from gel using gel extraction kit (Qiagen, Hilden, Germany) and ligated into a pGM-T vector (Tiangen Biotech, Beijing, China). Plasmid was extracted by Tiangen Tianperp Mini Plasmid kit. The plasmid was linearized overnight using NcoI (New England Biolabs, Nebraska, USA) and SalI (New England Biolabs) at 37°C. The cRNA probes were synthesized using Sp6 RNA polymerase (New England Biolabs) and T7 RNA polymerase (New England Biolabs) with digoxigenin-labeled rNTP (Roche Diagnostics, Rotkreuz, Switzerland).
In Situ Hybridization
Deparaffinized, dehydrated sections were incubated in 0.1 N HCl for 10 min, followed by antigen retrieval according standard procedures. The slides were allowed to hybridize with the IGHG1 antisense probes at 42°C for 20 h. The films were removed in 5 × SSC at 50°C. The slides were then washed in 2 × SSC plus 50% formamide and in 2 × SSC at 37°C. The slides were subsequently incubated with horse serum (1:100; Generay Biotech, Shanghai, China) and anti-digoxigenin-Ap Fab fragments (1:100; Roche Diagnostics) at room temperature for 1 h. The reaction was colorized with nitroblue tetrazolium/5-bromo-4-choloro-3-indolyl phosphate (NBT-BCIP; Promega, Madison, USA). For the IGHG1 cRNA probe, human spleen tissues were used as a positive control and sense probes as a negative control. For the C3 cRNA probe, human liver tissue was used as a positive control and sense probes as a negative control. In situ hybridization was performed on all of the 44 cases with paraformaldehyde-fixed samples, but not on the microarray.
Laser Capture Microdissection-Assisted RT-PCR
Frozen papillary thyroid cancer tissues were sectioned at 10 μm and mounted on membrane slides (Leica, Solms, Germany). The slides were fixed in 70% ethanol for 1 min, washed in DEPC-treated water (Generay Biotech). The slides were next stained using hematoxylin and dried in a fume hood. Groups of papillary thyroid cancer cells were identified by morphology and isolated from the tissue sections using a Leica laser capture microdissection system (Leica LMD 6000). Approximately 3000 cells were captured each time. Special care was taken not to isolate other cell types, lymphocytes in particular. Total RNA was extracted from the dissected cells using an RNeasy micro kit (Qiagen). Nested PCRs were performed using a Taq PCR kit (New England Biolabs). The primers are listed in Table 2. CD19, CD34, and CD68 were used to exclude contamination by B cells, endothelial cells, and macrophages, respectively. CK7 was used as a marker for papillary thyroid cancer cells. DEPC-treated water was used as a negative control. Raji cell line (B-cell lymphoma cell line) was used as a positive control for CD19, IGHG1, Igκ, recombination activating gene 1 (RAG1), RAG2, and activation-induced cytidine deaminase. Human fresh liver tissue was used as a positive control for CD34, CD68, and CK7. Amplification of the 18S gene was performed as internal control. PCR products were separated on 2% agarose gel by electrophoresis.
Evaluation of Ig Heavy Chain Expression
Immunohistochemistry was performed on the papillary thyroid cancer tissue microarray as described above. The primary antibody was polyclonal rabbit anti-human IgGγ (1:500). The IgGγ expression was semiquantified using the H-score as described by Finn et al.26 The intensity of staining was scored as follows: 1+, carcinoma cells stained light red or pink; 2+, carcinoma cells stained red; 3+, carcinoma cells selectively stained dark red. The H-score was based on the total percentage of positive cells and the intensity of the staining, where H=(%1+ × 1)+(%2+ × 2)+(%3+ × 3). A minimum of 100 cells were evaluated in calculating the H-score. Inter-examiner discrepancies were resolved by joint examination and mutual consensus of the two independent pathologists.
Statistical Analysis
The difference in H-scores between normal thyroid tissues and papillary thyroid cancer tissues, as well as the differences in IgG expression among the various clinicopathological features were assessed by the Mann–Whitney test. P<0.05 was considered statistically significant.27
Results
Immunoglobulin G and Complement Expression in Papillary Thyroid Cancer Tissues
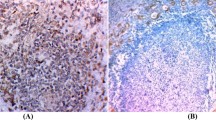
We studied the expression of IgGγ and Igκ in human papillary thyroid cancer tissues using polyclonal rabbit anti-human IgGγ antibodies, monoclonal mouse anti-human Igκ, respectively (Figure 1). Igγ and Igκ were both detected in papillary thyroid cancer tissues with immunoreactivity present in the cytoplasm of the cancer cells. Overall, 80% (35/44) of total samples studied, stained positive for IgGγ and 59% (26/44) were stained positive for Igκ. In contrast, IgG was not detected in normal tissues of the thyroid gland (Figure 2).
Immunohistochemistry and in situ hybridization on serial sections showing expression of IgG proteins and mRNA. Figures 1–5 and 7–10 are serial sections. The signals are localized to the cytoplasm. (1) Primary antibody: polyclonal rabbit anti-human IgGγ. (2) In situ hybridization show IGHG1 antisense probe. (3) Sense IGHG1 probe, no signal with a sense probe. (4) Primary antibody: mouse anti-human CD20. (5) Primary antibody: mouse anti-human CK. (6) Human spleen as a positive control for the IGHG1 antisense probe. (7) Primary antibody: mouse anti-human Igκ. (8) Primary antibody: mouse anti-human CD20. (9) Primary antibody: mouse anti-human CK. (10) PBS as a negative control. Scale bars, 50 μm.
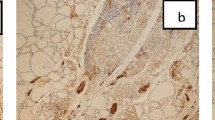
(a) Immunohistochemistry showing IgGγ (1), Igκ (2), C1q (3), C3c (4), and C4c (5) of normal thyroid tissues. No staining in the thyroid cells. Scale bars, 50 μm. (b) Immunohistochemistry with antibodies to IgG-specific Fc receptors on serial sections of papillary thyroid cancer tissues. Primary antibodies: (1) mouse anti-human CD16 (FcγRIII), (2) mouse anti-human CD32 (FcγRII), (3) mouse anti-human CD64 (FcγRI), (4) goat anti-human FcRn, and (5) PBS as a negative control. Scale bars, 50 μm. No positive staining is detectable in papillary thyroid cancer cells. No reactivity when PBS was used in place of the primary antibody. Immunohistochemistry signals are visualized with AEC, which gives a red color, and in situ hybridization signals are visualized with NBT-BCIP giving a purple-blue color. (c) Hematoxylin staining showing papillary thyroid cancer tissues before laser capture microdissection (1) and after laser capture microdissection (2). Scale bars, 100 μm.
We totally tested 20 samples for C1q, C3c, and C4c. In all, 80% were positive and the staining was located to the cytoplasm of papillary thyroid cancer cells. Applying immunohistochemistry on serial sections, we found that C1q, C3c, and C4c were colocalized with IgGγ in a part of the papillary thyroid cancer cells (Figure 3). But there were also cancer cells positive for IgGγ, but negative for complement proteins.
Immunohistochemistry on serial sections (Panels 1–6) demonstrating IgGγ (1), C1q (2), C3c (3), C4c (4), and CK (6) in the cytoplasm of papillary thyroid cancer cells. No CD20-positive B lymphocytes are detected (5). No immunoreactivity in the negative control (PBS) (7). Panels 8–10 show in situ hybridization of C3. (8) C3 antisense probe of papillary thyroid cancer. (9) C3 sense probe of papillary thyroid cancer. (10) Human liver as a positive control for C3 antisense probe. Scale bars, 50 μm.
Semiquantitative Evaluation of IgGγ Expression in Tissue Microarray
The H-score, based both on the total number of IgGγ-positive cells and the intensity of the staining, was calculated for each tissue sample on the tissue microarray. IgG expression was correlated with tumor size (Table 3). The larger the tumor was, the more IgGγ was expressed. The mean H-score in the papillary thyroid cancer samples was significantly higher than in normal thyroid tissues (Table 3). In addition, papillary thyroid cancer with local lymph node metastases had a significantly higher mean H-score than papillary thyroid cancer without lymph node metastases (Table 3). IgGγ expression was not correlated with age.
Papillary Thyroid Cancer Tissues Express IGHG1 Genes, but not Complement Genes
We performed in situ hybridization with an antisense probe against IGHG1 on 44 papillary thyroid cancer tissues. Positive signals were detected in 40 of 44 papillary thyroid cancer cases evaluated. The signals were localized to the cytoplasm (Figure 1). There were more cases showing positive in situ hybridization signals than those showing positive immunohistochemistry signals. Colocalization of IGHG1 mRNA signals and IgGγ was confirmed using serial sections (Figure 1). There were some cells that showed IGHG1-positive in situ hybridization signals, but no IgGγ immunohistochemistry-positive signals. In situ hybridization with an antisense probe for C3 did not yield any positive signal (Figure 3). The same probe to C3 yielded positive signal in the liver. Laser capture microdissection-assisted RT-PCR confirmed the presence of IGHG1 mRNA in papillary thyroid cancer. Igκ mRNA transcript was also successfully amplified from papillary thyroid cancer tissues (Figure 4).
Amplification of IGHG1, Igκ, RAG1, RAG2, and activation-induced cytidine deaminase mRNA transcripts in papillary thyroid cancer cells, using laser capture microdissection-assisted nested RT-PCR. Water was used as a negative control. CD19, CD34, and CD68 primers were used to exclude contamination by B cells, endothelial cells, and macrophages, respectively. CK7 primer was used as a cancer cell marker. Raji cell line was used as a positive control for CD19, IGHG1, Igκ, RAG1, RAG2, and activation-induced cytidine deaminase. Human liver tissue was used as a positive control for CD34, CD68, and CK7. 1, Raji; 2, papillary thyroid cancer cells; R, Dnase-treated RNA as template; C, cDNA as template; AID, activation-induced cytidine deaminase.
Gene Expression of RAG1, RAG2, and Activation-induced Cytidine Deaminase in Papillary Thyroid Cancer Tissues
We evaluated the expression of RAG1, RAG2, and activation-induced cytidine deaminase mRNA in papillary thyroid cancer cells. Using laser capture microdissection-assisted nested RT-PCR, RAG1, RAG2, and activation-induced cytidine deaminase mRNA transcripts were detected (Figure 4). We used CD19, CD34, and CD68 primers to exclude contamination by B cells, endothelial cells, and macrophages, respectively. CK7 primers were applied to identify epithelial cells. Our results showed that the dissected cells consisted only of papillary thyroid cancer cells.
Lack of IgG Fc Receptors in Papillary Thyroid Cancer Tissues
Immunostainings with antibodies to CD16, CD32, CD64, and FcRn were performed on papillary thyroid cancer tissue sections. None of the IgG-specific Fc receptors was expressed by papillary thyroid cancer cells (Figure 2). These findings exclude the possibility that the expression of IgG protein in papillary thyroid cancer cells was due to uptake by IgG Fc receptors.
Discussion
In this study we investigated the expression of IgG and complement proteins in papillary thyroid cancer. Papillary thyroid cancer represents the most prevalent type of thyroid cancer, with 80% of new cases of all thyroid cancers.12 Both IgG proteins and mRNA transcripts were identified in papillary thyroid cancer cells. Additional evidence supporting local IgG production was the detection of RAG1, RAG2, and activation-induced cytidine deaminase genes. Absence of any of the IgG-specific Fc receptors (CD16, CD32, CD64, and FcRn) ruled out the possibility that IgG protein expression had resulted from IgG uptake by receptors. The expression of RAG1 and RAG2 is consistent with previous studies showing a similar expression in colon, liver, lung, and breast cancer cell lines.6, 7 Activation-induced cytidine deaminase expression was also detected, which has, thus far, only been demonstrated in cancer cell lines, including the HeLa cell line and six breast cancer cell lines.7, 8 As lymphocytic infiltrate is frequently observed in papillary thyroid cancer, we had to take special care to avoid contamination by B cells in our tissue samples. In order to achieve this, we performed laser capture microdissection-assisted RT-PCR carefully isolating only papillary thyroid cancer cells, and we made use of several markers, including CD19, CD34, and CD68, to exclude contamination by B lymphocytes, endothelial cells, and macrophages, respectively.
Colocalization of IgG with C1q, C3c, and C4c was observed in papillary thyroid cancer tissues. As C1q is a component of the classical pathway to be activated upon the binding of IgG to an antigen, the colocalization of IgG and complement factors is most likely indicative of the presence of immune complexes. In terms of tumor pathogenesis, these immune complexes might consist of tumor surface antigens and IgG produced by the host against these antigens. Alternatively, they could be composed of host IgG against tumor antigens and locally produced anti-idiotypic IgG against the host antibody. The fact that tumor cells produce IgG themselves renders the latter option more plausible, as the formation of such immune complexes would confer a survival advantage to papillary thyroid cancer cells. Anti-idiotypic antibodies (ab2) are antibodies with specificity for epitopes in the hypervariable binding site (idiotopes) of the ‘original’ antibody (ab1) directed against the ‘original’ antigen.28 In tumor pathogenesis, ab1 is the antibody directed against tumor surface antigens and ab2 represents the anti-idiotypic antibody elicited against the hypervariable binding region of this ab1. On the basis of the co-expression of IgG and C1q, we would propose that locally produced anti-idiotypic IgG (ab2) captures antitumor antigen IgG (ab1), thus preventing induction of antibody-dependent cell-mediated cytotoxicity. By protecting tumor cells from cellular toxicity and cell death, IgG produced by tumor cells might indirectly promote tumor growth. Investigations are underway to study the function and specificity of tumor-produced IgG.
Another possible mechanism of how locally produced IgG could promote tumor growth is by acting as a direct growth factor. Previous studies have demonstrated that administration of anti-IgG antibodies in vitro and in mouse experiments inhibited growth of tumor cells.6, 10 Other growth-promoting factors have previously been identified in papillary thyroid cancer, including Fas ligand (FasL) and vascular endothelial growth factor (VEGF).29, 30 FasL is known to induce apoptosis in susceptible cancer cells and healthy cells. However, in papillary thyroid cancer cells binding of FasL to its receptor, Fas, has been found to promote proliferation.30 As both FasL and Fas are expressed in papillary thyroid cancer, FasL might exert its growth-promoting function through an autocrine signaling pathway. A similar autocrine loop might be involved in VEGF-induced cell survival in papillary thyroid cancer, given the co-expression of VEGF and its receptors.29 As we detected none of the, thus far, identified IgG receptors in papillary thyroid cancer tissues, however, involvement of an autocrine signaling pathway in IgG-mediated growth stimulation seems unlikely.
IgG might not only be involved in growth promoting of the primary tumor, but might also stimulate local invasion and metastasis. The fact that the H-score was higher in papillary thyroid cancer with local lymph node metastases than in those without is consistent with this assumption. Additional support includes the finding that increased IgG expression was also correlated with large tumor size.
Previous studies have shown that normal colorectal mucosa, adenomas, carcinomas, and human breast can produce complement proteins, as evidenced by the identification of C3, C4, and factor B mRNA transcripts by in situ hybridization.20, 31 In papillary thyroid cancer, however, we found no evidence for local complement production, as only the protein of C3c without its corresponding mRNA transcript was detected. This indicates that the presence of these complement proteins was not due to local production, but the result of deposition from the serum and in situ activation. As expression of C1q, C3c, and C4c was detected in this study, and the expression of C3d, C4d, C5, and C5b-C9 in papillary thyroid cancer by other groups, it appears that locally produced IgG is not capable of fully preventing the activation of the complement system.11, 21 This could be explained by the fact that the complement system can also be activated by the alternative and lectin pathway. An additional mechanism protecting cancer cells from destruction by the complement system is through the expression of mCRPs on cancer cell membranes. Papillary thyroid cancer cells express mCRPs, including CD46, CD53, CD55, and CD59, as well as the soluble CRP, vitronectin.11, 21 In this respect, papillary thyroid cancer cells do not appear to differ significantly from the normal human thyroid follicular cells showing a similar expression of mCRPs.21, 32
In conclusion, we established that papillary thyroid cancer cells are capable of producing IgG. Given its increased expression in papillary thyroid cancer with large tumor size and local lymph node metastases, it is suggested that IgG appeared to be positively related to the growth and metastasis of papillary thyroid cancer cells. Its colocalization with C1q, C3c, and C4c suggests the presence of immune complexes. The formation of such immune complexes might protect the cancer cells from destruction by the complement system, thereby favoring tumor proliferation. This would provide a new insight into the mechanism of tumorigenesis and new clues for tumor therapy. The exact mechanisms of the observed phenomenon warrant further investigation.
References
Terry WD, Fahey JL . Subclasses of human gamma globulin based on differences in the heavy polypeptide chains. Science 1964;146:400–401.
Niu N, Zhang J, Guo Y, et al. Expression and distribution of immunoglobulin G and its receptors in the human nervous system. Int J Biochem Cell Biol 2011;43:556–563.
Zhang S, Mao Y, Huang J, et al. Immunoglobulin gene locus events in epithelial cells of lactating mouse mammary glands. Cell Mol Life Sci 2010;67:985–994.
Niu N, Zhang J, Sun Y, et al. Expression and distribution of immuglobulin G and its receptors in an immune privileged site: the eye. Cell Mol Life Sci 2011;68:2481–2492.
Zhao Y, Liu Y, Chen Z, et al. Immunoglobulin G (IgG) expression in human umbilical cord endothelial cells. J Histochem Cytochem 2011;59:474–488.
Qiu X, Zhu X, Zhang L, et al. Human epithelial cancers secrete immunoglobulin G with unidentified specificity to promote growth and survival of tumor cells. Cancer Res 2003;63:6488–6495.
Chen Z, Gu J . Immunoglobulin G expression in carcinomas and cancer cell lines. FASEB J 2007;21:2931–2938.
Babbage G, Ottensmeier CH, Blaydes J, et al. Immunoglobulin heavy chain locus events and expression of activation-induced cytidine deaminase in epithelial breast cancer cell lines. Cancer Res 2006;66:3996–4000.
Chen Z, Huang X, Ye J, et al. Immunoglobulin G is present in a wide variety of soft tissue tumors and correlates well with proliferation markers and tumor grades. Cancer 2010;116:1953–1963.
Lee G, Chu RA, Ting HH . Preclinical assessment of anti-cancer drugs by using RP215 monoclonal antibody. Cancer Biol Ther 2009;8:161–166.
Lucas SD, Kaelsson-Parra A, Nilsson B, et al. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Hum Pathol 1996;27:1329–1335.
Hay ID, McConahey WM, Goellner JR . Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic’s experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc 2002;113:241–260.
Sculumberger MJ . Papillary and follicular thyroid carcinoma (review). N Engl J Med 1998;338:297–306.
Haymart MR . Understanding the relationship between age and thyroid cancer. Oncologist 2009;14: 216–221.
Kuriakose MA, Hicks Jr WL, Loree TR, et al. Risk group-based management of differentiated thyroid carcinoma. J R Coll Surg Edinb 2001;46:216–223.
Andersen PE, Kinsella J, Loree TR, et al. Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg 1995;170:467–470.
Jones MK . Management of papillary and follicular thyroid cancer. J R Soc Med 2002;95:325–326.
Zipfel PF, Skerka C . Complement regulators and inhibitory proteins. Nat Rev Immunol 2009;9:729–740.
Witte DP, Welch TR, Beischel LS . Detection and cellular localization of human C4 gene expression in the renal tubular epithelial cells and other extrahepatic epithelial sources. Am J Pathol 1991;139:717–724.
Andoh A, Fujiyama Y, Sakmoto H, et al. Detection of complement C3 and factor B gene expression in normal colorectal mucosa, adenomas and carcinomas. Clin Exp Immunol 1998;111:477–483.
Yamakawa M, Yamada K, Tsuge T, et al. Protection of thyroid cancer cells by complement-regulatory factors. Cancer 1994;73:2808–2817.
Stover C . Dual role of complement in tumour growth and metastasis (review). Int J Mol Med 2010;25:307–313.
Markiewski MM, Lambris JD . Is complement good or bad for cancer patients? A new perspective on an old dilemma. Trends Immunol 2009;30:286–292.
Raghavan M, Bjorkman PJ . Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol 1996;12:181–220.
Roopenian DC, Akilesh S . FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007;7:715–725.
Finn RS, Press MF, Dering J, et al. Estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor expression and benefit from lapatinib in a randomized trial of paclitaxel with lapatinib or placebo as first-line treatment in HER2-negative or unknown metastatic breast cancer. J Clin Oncol 2009;27:3908–3915.
Li M, Feng DY, Ren W, et al. Expression of immunoglobulin kappa light chain constant region in abnormal human cervical epithelial cells. Int J Biochem Cell Biol 2004;36:2250–2257.
Pan Y, Yuhasz SC, Amzel LM . Anti-idiotypic antibodies: biological function and structural studies. FASEB J 1995;9:43–49.
Vieira JM, Santos SC, Espadinha C, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors in thyroid carcinomas of follicular origin: a potential autocrine loop. Eur J Endocrinol 2005;153:701–709.
Mitsiades CS, Poulaki V, Fanourakis G, et al. Fas signaling in thyroid carcinoma is diverted from apoptosis to proliferation. Clin Cancer Res 2006; 12:3705–3712.
Laufer J, Oren R, Goldberg I, et al. Local complement genes expression in the mammary gland: effect of gestation and inflammation. Pediatr Res 1999;46:608–612.
Tandon N, Morgan BP, Weetman AP . Expression and function of membrane attack complex inhibitory proteins on thyroid follicular cells. Immunology 1992;75:372–377.
Acknowledgements
We acknowledge Professor Min Su of Shantou University Medical College for providing papillary thyroid cancer tissues. This work was supported by grants (81030033, 30971150 to JG, 81001199 to ZC, and 30950110335 to CK) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Qiu, Y., Korteweg, C., Chen, Z. et al. Immunoglobulin G expression and its colocalization with complement proteins in papillary thyroid cancer. Mod Pathol 25, 36–45 (2012). https://doi.org/10.1038/modpathol.2011.139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.139
Keywords
This article is cited by
-
Current insights into the expression and functions of tumor-derived immunoglobulins
Cell Death Discovery (2021)
-
IgG silencing induces apoptosis and suppresses proliferation, migration and invasion in LNCaP prostate cancer cells
Cellular & Molecular Biology Letters (2016)
-
Immunoglobulin G Expression in Human Sperm and Possible Functional Significance
Scientific Reports (2016)
-
Identification of Liver Epithelial Cell-derived Ig Expression in μ chain-deficient mice
Scientific Reports (2016)
-
Identification of Genes Associated with Smad3-dependent Renal Injury by RNA-seq-based Transcriptome Analysis
Scientific Reports (2015)