Abstract
The purpose of this study was to determine whether p53 protein expression in tumor-stromal fibroblasts forming fibrotic foci is a significant outcome predictor, similar to p53 protein expression in tumor-stromal fibroblasts not forming fibrotic foci, and whether the combined assessment of p53 expression in tumor-stromal fibroblasts forming and not forming fibrotic foci served as an important outcome predictor among 1039 patients with invasive ductal carcinoma of the breast. We analyzed the outcome predictive power of the Allred score risk classification for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci using multivariate analyses with well-known clinicopathological factors. The Allred score risk classifications for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci were superior to the Allred scores for p53 in tumor-stromal fibroblasts not forming fibrotic foci alone for accurately predicting the tumor-related death of patients with invasive ductal carcinoma when examined using multivariate analyses. The Allred score risk classification for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci significantly increased the hazard rates for tumor recurrence and tumor-related death independent of the UICC pTNM stage in the multivariate analyses. These results indicated that the Allred score risk classification based on the combined assessment of p53 expression in tumor-stromal fibroblasts forming and not forming fibrotic foci is a very useful outcome predictor among patients with invasive ductal carcinoma.
Similar content being viewed by others
Main
Along with others, we have already reported that a fibrotic focus, a characteristic histological feature of tumor stroma, is a very useful histological tumor-stromal indicator for accurately predicting the outcome of patients with invasive ductal carcinoma (IDC),1, 2, 3, 4, 5 and the proliferative activity of tumor-stromal fibroblasts forming and not forming fibrotic foci has a very important function in nodal metastasis and distant organ metastasis by IDCs.6, 7 Because it has recently been reported that the gene expression profile and protein expression profile of the tumor stroma have a very important function in tumor progression in carcinoma8, 9 and that the interactions between tumor cells and stromal cells also are very important in tumor progression in carcinomas,10, 11 these findings strongly suggest that the tumor stroma has a significant function in tumor progression in IDCs. Mutations of the p53 tumor suppressor gene have been described in the stromal fibroblasts of breast and prostate carcinomas in humans and experimental animals,12, 13, 14 and p53 mutations in breast cancer stromal cells have been reported to be closely associated with nodal metastasis.15 However, some studies have reported that p53 mutations are not observed in the tumor stroma of breast cancer,16, 17 and the possibility of technical problem, eg polymerase chain reaction artifacts for the p53 gene abnormality, has been suggested by Campbell et al.18 We recently showed that p53 expression in tumor-stromal fibroblasts not forming fibrotic foci was a very important outcome predictor for IDC patients who had or had not received neoadjuvant therapy.19, 20 On the basis of the above findings, the p53 status of tumor-stromal fibroblasts not forming fibrotic foci probably has a very important function in tumor progression in IDCs.
We also previously reported that our newly devised grading system for lymph vessel tumor emboli is a very useful histological grading system for accurately predicting the outcome of patients with IDC who have not received neoadjuvant therapy; furthermore, this grading system can be used to classify the prognosis of IDC patients with lymph vessel invasion into low-risk, intermediate-risk, and high-risk groups.21 In addition, we recently confirmed that this grading system for lymph vessel tumor emboli was a very important outcome predictor for patients with IDC in a different patient group.22
The purpose of this study was to determine whether the combined assessment of p53 expression in tumor-stromal fibroblasts forming and not forming fibrotic foci served as an important outcome predictor among patients with IDC of the breast using multivariate analyses with well-known prognostic factors and our grading system for lymph vessel tumor emboli. The results indicated that a score classification based on the combined assessment of p53 expression in tumor-stromal fibroblasts forming and not forming fibrotic foci was a very useful outcome predictor among patients with IDC of the breast.
Materials and methods
Cases
The subjects of this study were 1039 consecutive patients with IDC of the breast who did not receive neoadjuvant therapy and who were surgically treated at the National Cancer Center Hospital between January 2000 and December 2005 (almost the same case series as that used in our previous study).19, 22 The IDCs were diagnosed preoperatively using needle biopsy, aspiration cytology, a mammography, or ultrasonography. All the patients were Japanese women, ranging in age from 23 to 72 years (median, 55 years). All had a solitary lesion; 497 patients were premenopausal and 542 were postmenopausal. A partial mastectomy had been performed in 455 patients, and a modified radical mastectomy had been performed in 584. A level I and level II axillary lymph node dissection had been performed in all the patients, and a level III axillary lymph node dissection had been performed in some of the patients with IDC.
Of the 1039 patients, 873 received adjuvant therapy, consisting of chemotherapy in 218 patients, endocrine therapy in 281 patients, and chemoendocrine therapy in 374 patients. The chemotherapy regimens used were anthracycline-based with or without taxane and non-anthracycline-based, and the endocrine therapy regimens consisted of tamoxifen with or without a gonadotropin-releasing hormone agonist, tamoxifen, with or without an aromatase inhibitor, an aromatase inhibitor alone, or a gonadotropin-releasing hormone agonist alone. No cases of inflammatory breast cancer were included in this series. All the tumors were classified according to the pathological UICC-TNM (pTNM) classification.23 The protocol of this study (20-112) was reviewed by the institutional review board of the National Cancer Center.
For the pathological examination, we fixed the surgically resected specimens in 10% formalin, and the size and gross appearance of the tumors were recorded. The tumor size was confirmed by comparison with the tumor size on the histological slides; if more than one invasive focus was present, the size of the largest invasive focus was recorded as the invasive tumor size, based on a previously reported definition for determining the size of microinvasion in IDC with multiple microinvasive foci23 in this study.
Histological Examination
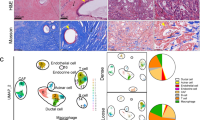
Serial sections of each tumor area were cut from paraffin blocks. One section from each tumor was stained with hematoxylin and eosin and was examined histologically to confirm the diagnosis, and another section was subjected to immunohistochemistry. The following eight histological factors and the grading system for lymph vessel tumor emboli21, 22 were evaluated: (1) invasive tumor size (≤20, >20 to ≤50, >50 mm); (2) histological grade (1, 2, 3);24 (3) tumor necrosis (absent, present);25 (4) fibrotic focus (absent, fibrotic focus diameter ≤8 mm, fibrotic focus diameter >8 mm) (Figure 1);1, 2 (5) blood vessel invasion (absent, present); (6) adipose tissue invasion (absent, present); (7) skin invasion (absent, present); and (8) muscle invasion (absent, present).
Invasive ductal carcinomas with fibrotic foci (a–d). (a) A fibrotic focus measuring 6.4 × 3.3 mm is visible within the tumor (panoramic view, arrows). The fibrotic focus shows a scar-like feature and is surrounded by invasive ductal carcinoma cells. (b) The fibrotic focus area consists mainly of fibroblasts arranged in a storiform pattern. (c) A fibrotic focus measuring 10.2 × 7.3 mm is visible within the tumor (panoramic view, arrows). The fibrotic focus has a fibrosclerotic core and is surrounded by invasive ductal carcinoma cells. Small residual tumor islands are present within the fibrotic focus. (d) The fibrotic focus consists of fibroblasts and hyalinized collagen fibers in a storiform arrangement.
Immunohistochemistry
Immunohistochemical staining for estrogen receptors, progesterone receptors, p53, and HER2 products was performed using an autoimmunostainer (Optimax Plus; BioGenex, San Ramon, CA, USA). The antigen retrieval device for Optimax Plus was an autoclave, and each specimen was immersed in citrate buffer and incubated at 121°C for 10 min. Immunoperoxidase staining was performed using a labeled streptavidin biotin staining kit (BioGenex) according to the manufacturer's instructions. The antibodies used were the anti-estrogen receptor mouse monoclonal antibody ER88 (BioGenex), the anti-progesterone receptor mouse monoclonal antibody PR88 (BioGenex), the anti-HER2 mouse monoclonal antibody CB11 (BioGenex), and the p53 mouse monoclonal antibody DO7 (Dako, Glostrup, Denmark). ER88, PR88, and CB11 were previously diluted, and DO7 was applied at a dilution of 1:100. After immunostaining, the sections were counterstained with hematoxylin. Sections of the IDCs that were positive for estrogen receptor, progesterone receptor, HER2, and p53 were used each time as a positive control. As a negative control, the primary antibody was replaced with normal mouse immunoglobulin.
Assessment of ER, PR, p53, and HER2 Expression
Slides of the tumor cells immunostained for estrogen receptor, progesterone receptor, and p53 were scored using the Allred scoring system, as described previously,26, 27, 28 and the Allred scores for estrogen receptor, progesterone receptor, and p53 expression in the tumor cells were classified into the following three categories19: (1) Allred score for estrogen receptor in tumor cells (0 or 2, 3–6, and 7 or 8); (2) Allred score for progesterone receptor in tumor cells (0 or 2, 3–6, and 7 or 8); and (3) Allred scores for p53 in tumor cells (0 or 2 or 3, 4–6, and 7 or 8). We modified the Allred scoring system to assess the nuclear expression of p53 in the tumor-stromal fibroblasts forming and not forming fibrotic foci,19, 20 and the Allred scores for p53 expression in tumor-stromal fibroblasts forming and not forming fibrotic foci were classified into the following categories: (1) Allred scores for p53 in tumor-stromal fibroblasts forming fibrotic foci (0, 2, 3, and 4–8); and (2) Allred scores for p53 in tumor-stromal fibroblasts not forming fibrotic foci (0 or 2, 3, and 4–8) (Figures 2 and 3). Of the 1039 IDCs, 373 IDCs had fibrotic foci; we could not assess the Allred scores for p53 in tumor-stromal fibroblasts forming a fibrotic focus in 97 of the 373 IDCs with fibrotic foci because the immunohistochemistry examinations for these specimens were performed using tumor tissue sections that did not contain a fibrotic focus at the time of routine examination. The HER2 status of the tumor cells was semiquantitatively scored on a scale of 0–3 according to the level of HER2 protein expression,29 and it was classified into three categories: 0 or 1, 2, and 3.
Tumor-stromal fibroblasts forming (a, c, e) and not forming a fibrotic focus (b, d, f). A fibrotic focus consists of tumor-stromal fibroblasts and hyalinized collagen fibers (a and c) and many tumor-stromal fibroblasts show a moderately intense nuclear staining pattern for p53. The Allred score for p53 in these tumor-stromal fibroblasts forming a fibrotic focus is 7 (intensity score, 2; proportion score, 5) (e). Carcinoma cells invade in irregular-shaped nests with a tubular structure (b) and tumor-stromal fibroblasts with oval nuclei not forming a fibrotic focus are seen (d). Many tumor-stromal fibroblasts not forming a fibrotic focus show a faint, moderate or strong intense nuclear staining pattern for p53, whereas tumor cells showing a faint intense nuclear staining pattern for p53 are visible (f). The Allred score for p53 in these tumor-stromal fibroblasts not forming a fibrotic focus is 8 (intensity score, 3; proportion score, 5).
Patient Outcome and Statistical Analysis
Survival was evaluated using a median follow-up period of 52 months (range: 18–102 months) until February 2009. Of the 1039 IDC patients, 910 patients were alive and well, 129 had developed tumor recurrences, and 58 had died of their disease. The tumor recurrence-free survival and overall survival periods were calculated using the time of surgery as the starting point. Tumor relapse was considered to have occurred whenever evidence of metastasis was found.
The Mann–Whitney test was used to compare the Allred scores for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci, and the correlation analyses were performed using Cochran-Mantel-Haenszel statistics.
We analyzed the outcome predictive power of the eight histological factors, the grading system for lymph vessel tumor emboli;21, 22 the Allred scores for estrogen receptor; progesterone receptor, and p53 in tumor cells; the category of HER2 expression in tumor cells; the Allred score risk classification for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci, adjuvant therapy (yes or no); age (≤39 years and >39 years); and the UICC-pathological nodal status (N factor, ie, no nodal metastasis, N0; 1–3 nodal metastases, N1; 4–9 nodal metastases, N2; and 10 or more nodal metastases, N3)23 for tumor recurrence, and tumor-related death in univariate analyses using the Cox proportional hazard regression model. The factors significantly associated with outcome in the univariate analyses were then entered together into the multivariate analyses using the Cox proportional hazard regression model according to the UICC pTNM stage. The case-wise and step-down method was applied until all the remaining factors were significant at a P-value of below 0.05. Because fewer than 10 tumor-related deaths occurred among the UICC stage I IDC patients (Table 2), it was impossible to perform multivariate analyses for tumor-related death in this group. All the analyses were performed using Statistica for Windows software (StatSoft, Tulsa, OK, USA).
Results
Allred Scores for p53 in Tumor-Stromal Fibroblasts Forming and Not Forming Fibrotic Foci
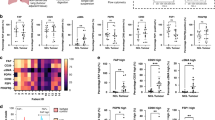
Although a significant association was observed between the Allred scores for p53 in tumor-stromal fibroblasts forming and those not forming fibrotic foci (P<0.001; Figure 4a), the latter value (mean value, 2.2; standard deviation, 2.1) was significantly higher than the former (mean value, 1.6; standard deviation, 2.0; P=0.001). The Allred scores for p53 in tumor-stromal fibroblasts forming fibrotic foci were also significantly associated with the fibrotic focus diameter, and in IDCs with a fibrotic focus diameter >8 mm, the number of IDCs with Allred scores of 4–8 for p53 in tumor-stromal fibroblasts forming fibrotic foci was larger than that of IDCs with Allred scores of 0, 2, or 3 for p53 in tumor-stromal fibroblasts forming fibrotic foci (Figure 4b).
(a) Associations between the Allred scores for p53 in tumor -stromal fibroblasts forming and not forming fibrotic foci; the scores were significantly associated with each other (P<0.001). (b) Associations between the Allred scores for p53 in tumor-stromal fibroblasts forming fibrotic foci and the diameter of the fibrotic foci. Invasive ductal carcinomas with fibrotic foci >8 mm in diameter had a significantly higher Allred score for p53 in tumor-stromal fibroblasts forming fibrotic foci than those with fibrotic foci ≤8 mm in diameter (P=0.006).
Allred Score Risk Classification for p53 in Tumor-Stromal Fibroblasts Forming and not Forming Fibrotic Foci in Patients with Invasive Ductal Carcinoma with and without Fibrotic Foci
We devised an Allred score risk classification for p53 in tumor-stromal fibroblasts in IDCs based on the combined Allred scores for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci (Table 1). This classification was successfully used to classify IDC patients with or without fibrotic foci into three risk classes (low risk, intermediate risk, and high risk) according to the ratios for tumor recurrence and tumor-related death (Table 2; Figure 5). Among the UICC pTNM stage I IDC patients, the patients in the intermediate- and high-risk classes showed a significantly higher tumor recurrence rate than the patients in the low-risk class (Table 2). Among the UICC pTNM stage II IDC patients, the tumor recurrence rate and the mortality rate for each risk class were significantly increased according to the risk classes of the classification (Table 2). Among the UICC pTNM stage III IDC patients, the patients in the intermediate-risk class showed a significantly higher tumor recurrence rate and mortality rate than the patients in the low-risk class, and the patients in the high-risk class showed a marginally significantly higher tumor recurrence rate and a significantly higher mortality rate than the patients in the intermediate-risk class (Table 2).
Disease-free survival curves and overall survival curves of invasive ductal carcinoma (IDC) patients overall (a and b) according to the Allred score risk classification for p53 in tumor-stromal fibroblasts forming and not forming a fibrotic focus (FF). The disease-free survival time (a) and the overall survival time (b) of the IDC patients significantly decrease with the risk class of the Allred score risk classification for p53 in tumor-stromal fibroblasts forming and not forming FF.
Overall, the Allred score risk classification for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci (trend hazard rate, 2.9; trend 95% confidence interval, 1.6–5.2; P-value, <0.001) was superior to the Allred scores for p53 in tumor-stromal fibroblasts not forming fibrotic foci alone (trend hazard rate, 1.5; trend 95% confidence interval, 0.8–2.6; P-value, 0.172) for accurately predicting tumor-related death among patients with IDC, as shown in a multivariate analysis.
Factors Significantly Associated with Tumor Recurrence and Tumor-Related Death
Among the patients with UICC pTNM stage I IDC, an intermediate-risk class (hazard rate, 6.2; 95% confidence interval, 2.1–18.5; P-value, 0.001) and a high-risk class (hazard rate, 11.6; 95% confidence interval, 2.1–63.8; P-value, 0.005) for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci and a histological grade of 3 (hazard rate, 2.9; 95% confidence interval, 1.1–7.6; P-value, 0.034) significantly increased the hazard rates for tumor recurrence in a multivariate analysis.
Among the patients with UICC pTNM stage II IDC, an intermediate-risk class and a high-risk class for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci significantly increased the hazard rates for tumor recurrence and tumor-related death in the multivariate analyses (Table 3). Grades 2 and 3 lymph vessel tumor emboli and the presence of blood vessel invasion significantly increased the hazard rates for tumor recurrence in the multivariate analysis (Table 3). A UICC pN1 category and a fibrotic focus diameter >8 mm significantly increased the hazard rates for tumor-related death and an Allred score of 7 or 8 for the progesterone receptors in the tumor cells significantly decreased the hazard rate for tumor-related death in the multivariate analyses (Table 3).
Among the patients with a UICC pTNM stage III IDC, an intermediate-risk class and a high-risk class for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci, grade 3 lymph vessel tumor emboli and a UICC pN3 category significantly increased the hazard rates for tumor recurrence and tumor-related death in the multivariate analysis (Table 4). A fibrotic focus diameter >8 mm significantly increased the hazard rate for tumor recurrence and an Allred score of 7 or 8 for estrogen receptor in the tumor cells significantly decreased the hazard rate for tumor-related death in the multivariate analysis (Table 4).
Discussion
This study clearly showed that the values of the Allred scores for p53 in tumor-stromal fibroblasts not forming fibrotic foci were significantly higher than those in tumor-stromal fibroblasts forming fibrotic foci. Fibrotic foci are fibrotic scar-like lesions that mainly consist of tumor-stromal fibroblasts admixed with various numbers of tumor cells; some fibrotic foci do not contain any tumor cells.1, 2 In contrast, tumor-stromal fibroblasts not forming fibrotic foci commonly admix with many tumor cells that show stromal invasion. This difference strongly suggests that the tumor cell–stromal cell interaction occurs more frequently in the outer area of a fibrotic focus than in the inner area of a fibrotic focus within IDCs,10, 11 probably resulting in the higher Allred scores for p53 in tumor-stromal fibroblasts not forming fibrotic foci. However, the Allred scores for p53 in tumor-stromal fibroblasts forming fibrotic foci were significantly associated with those for p53 in tumor-stromal fibroblasts not forming fibrotic foci. Thus, the tumor cell–stromal cell interaction probably occurs more frequently in IDCs with fibrotic foci than in IDCs without fibrotic foci.
We and others have already reported that the fibrotic focus diameter is a significant outcome predictor among patients with IDC who have fibrotic foci,1, 2, 3, 4, 5 and our previous study showed that a fibrotic focus diameter of greater than 8 mm, similar to the Allred score for p53 in tumor-stromal fibroblasts not forming a fibrotic focus, was a significant outcome predictor for patients with IDC independent of the UICC pTNM stage.19 In this study, a fibrotic focus diameter was also a significant outcome predictor for IDC patients of UICC pTNM stage II and IDC patients of UICC pTNM stage III, and IDCs with fibrotic foci greater than 8 mm in diameter showed a significantly higher Allred score for p53 in tumor-stromal fibroblasts forming fibrotic foci than IDCs with fibrotic foci of 8 mm or less in diameter. Thus, one can conclude that p53-expressing tumor-stromal fibroblasts located in both the inner and outer regions of fibrotic foci heighten the malignant potential of IDCs, probably accounting for the prognostic value of the fibrotic focus diameter. In addition, the grading system for lymph vessel tumor emboli significantly increased the hazard rates for tumor recurrence or tumor-related death in multivariate analyses performed for IDC patients with UICC pTNM stage II and UICC stage III. Therefore, the fibrotic focus diameter and the grading system for lymph vessel tumor emboli are likely to be very important histological outcome predictors for patients with IDC.
The results of this study clearly show that the Allred score risk classification for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci had a greater outcome predictive power than the Allred scores for p53 in tumor-stromal fibroblasts not forming fibrotic foci alone. Furthermore, the Allred score risk classification for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci is a very important outcome predictor for patients with IDC and an intermediate-risk or high-risk classification significantly increased the hazard rates for tumor recurrence and tumor-related death independent of the UICC pTNM stage in multivariate analyses that included well-known prognostic factors. Thus, we can conclude that the Allred score risk classification based on the Allred score for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci appears to be an excellent histological predictor of outcome among patients with IDC with or without fibrotic foci. However, as we could not analyze the outcome predictive power of the Allred score risk classification for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci among patients with IDC according to the types of adjuvant therapy (chemotherapy, endocrine therapy, and chemoendocrine therapy) in detail, the predictive power of the Allred score risk classification for p53 in tumor-stromal fibroblasts forming and not forming fibrotic foci should be analyzed separately among IDC patients treated with chemotherapy, endocrine therapy, and chemoendocrine therapy in the future.
In this study, we did not investigate the associations of the Allred scores for p53 with the presence of p53 gene abnormalities in tumor-stromal fibroblasts. Although p53 mutations in tumor-stromal fibroblasts are relatively common among primary breast cancers and other cancers and have been reported to exert a positive effect on cancer growth,12, 13, 14, 15 some studies have not shown any p53 mutations in the tumor-stroma of breast cancer.16, 17, 18 We have already reported that fibroblasts forming fibrotic foci show significantly higher proliferative activities than those not forming fibrotic foci and found that no significant association exists between the proliferative activity of fibroblasts forming fibrotic foci and the fibrotic foci diameter.7 In contrast, the Allred scores for p53 in tumor-stromal fibroblasts forming fibrotic foci were significantly lower than the Allred scores for p53 in tumor-stromal fibroblasts not forming fibrotic foci, and a significant association between the increase in the Allred scores for p53 in tumor-stromal fibroblasts forming fibrotic foci and the fibrotic foci diameter was observed in this study. Thus, although the mechanism that increases the malignant potential of IDCs through the expression of p53 in tumor-stromal fibroblasts should be investigated from the viewpoint of p53 gene abnormalities, p53 immunoreactivity in tumor-stromal fibroblasts produced by tumor cell–stromal cell interactions inside and outside fibrotic foci might in fact reflect specific reactive changes other than the proliferative activity of fibroblasts forming fibrotic foci within the stroma that might be correlated with the prognosis.
In conclusion, this is the first study to show clearly that p53 expression in tumor-stromal fibroblasts forming and not forming fibrotic foci is strongly associated with the outcome of IDC patients. Because p53 expression in tumor-stromal fibroblasts forming and not forming fibrotic foci might be important in tumor progression in IDCs, p53 expression could be a very important target for tumor gene therapy for IDCs, suppressing tumor cell–stromal cell interactions arising from p53 gene abnormalities or p53-related tumor microenvironment reactions.
References
Hasebe T, Tsuda H, Hirohashi S, et al. Fibrotic focus in infiltrating ductal carcinoma of the breast: a significant histopathological prognostic parameter for predicting the long-term survival of the patients. Breast Cancer Res Treat 1998;49:195–208.
Hasebe T, Sasaki S, Imoto S, et al. Prognostic significance of fibrotic focus in invasive ductal carcinoma of the breast: a prospective observational study. Mod Pathol 2002;15:502–516.
Colpaert C, Vermeulen PB, van Beest P, et al. Intratumoral hypoxia resulting in the presence of a fibrotic focus is an independent predictor of early distant relapse in lymph node-negative breast cancer patients. Histopathology 2001;39:416–425.
Baak JP, Colpaert CG, van Diest PJ, et al. Multivariate prognostic evaluation of the mitotic activity index and fibrotic focus in node-negative invasive breast cancers. Eur J Cancer 2005;41:2093–2101.
Van den Eynden GG, Colpart CG, Couveland A, et al. A fibrotic focus is a prognostic factor and a surrogate marker for hypoxia and (lymph) angiogenesis in breast cancer: review of the literature and proposal on the criteria of evaluation. Histopathology 2007;51:440–451.
Hasebe T, Sasaki S, Imoto S, et al. Proliferative activity of intratumoral fibroblasts is closely correlated with lymph node and distant organ metastases of invasive ductal carcinoma of the breast. Am J Pathol 2000;156:1701–1710.
Hasebe T, Sasaki S, Imoto S, et al. Highly proliferative fibroblasts forming fibrotic focus govern metastasis of invasive ductal carcinoma of the breast. Mod Pathol 2001;14:325–337.
Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 2008;14:518–527.
Singer CF, Gschwantler-Kaulich D, Fink-Retter A, et al. Differential gene expression profile in breast cancer-derived stromal fibroblasts. Breast Cancer Res Treat 2008;110:273–281.
Hasegawa M, Furuya M, Kasuya Y, et al. CD151 dynamics in carcinoma–stroma interaction: integrin expression, adhesion strength and proteolytic activity. Lab Invest 2007;87:882–892.
Studebaker AW, Storci G, Werbeck JL, et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res 2008;68:9087–9095.
Kurose K, Gilley S, Matsumoto PH, et al. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinoma. Nat Genet 2002;32:355–357.
Hill R, Song Y, Cardiff RD, et al. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell 2006;123:1001–1011.
Bierie B, Moses HL . Under pressure: stromal fibroblasts change their ways. Cell 2005;123:985–987.
Patocs A, Zhang L, Xu Y, et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med 2007;357:2543–2551.
Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004;6:17–32.
Lebret SC, Newgreen DF, Thompson EW, et al. Induction of epithelial to mesenchymal transition in PMC42-LA human breast carcinoma cells by carcinoma-associated fibroblast secreted factors. Breast Cancer Res 2007;9:R19.
Campbell IG, Qiu W, Polyak K, et al. Breast-cancer stromal cells with TP53 mutations. N Engl J Med 2008;10:1634–1635.
Hasebe T, Okada N, Tamura N, et al. p53 expression in tumor-stromal fibroblasts is closely associated with the outcome of patients with invasive ductal carcinoma. Cancer Sci 2009;100:2101–2108.
Hasebe T, Tamura N, Okada N, et al. p53 expression in tumor-stromal fibroblasts is closely associated with the nodal metastasis and outcome of patients with invasive ductal carcinoma who received neoadjuvant therapy. Hum Pathol 2010;41:262–270.
Hasebe T, Yamauchi C, Iwasaki M, et al. Grading system for lymph vessel tumor emboli for prediction of the outcome of invasive ductal carcinoma of the breast. Hum Pathol 2008;39:427–436.
Hasebe T, Okada N, Iwasaki M, et al. Grading system for lymph vessel tumor emboli: significant outcome predictor for invasive ductal carcinoma of the breast. Hum Pathol 2010 (in press).
Sobin LH, Wittekind Ch, (eds). TNM Classification of Malignant Tumors 6th edn. Wiley-Liss: Geneva, 2002, pp 131–141.
Bloom HJG, Richardson WW . Histological grading and prognosis in breast cancer. Br J Cancer 1957;11:359–377.
Gilchrist KW, Gray R, Fowble B, et al. Tumor necrosis is a prognostic predictor for early recurrence and death in lymph node-positive breast cancer: a 10-year follow-up study of 728 Eastern Cooperative Oncology Group patients. J Clin Oncol 1993;11:1929–1935.
Harvey JM, Clark GM, Osborne K, et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 1999;17:1474–1481.
Mohsin S, Weiss H, Havighurst T, et al. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol 2004;17:1545–1554.
Allred DC, Clark GM, Elledge R, et al. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst 1993;85:200–206.
Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007;131:18–43.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) (C) (21590393) from the Japan Society for the Promotion of Science and was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan (20-16, H21-006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hasebe, T., Iwasaki, M., Akashi-Tanaka, S. et al. p53 expression in tumor-stromal fibroblasts forming and not forming fibrotic foci in invasive ductal carcinoma of the breast. Mod Pathol 23, 662–672 (2010). https://doi.org/10.1038/modpathol.2010.47
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.47