Abstract
LIN28 has been shown to have an important role in primordial germ cell development and malignant transformation of germ cells in mouse. In this study, we examined the immunohistochemical profile of LIN28 in 131 primary human extragonadal germ cell tumors (central nervous system (CNS) 76, mediastinum 17, sacrococcygeal region 30, pelvis 3, vagina 2, liver 1, omentum 1, and retroperitoneum 1), including the following tumors and/or components: 57 seminomas/germinomas, 10 embryonal carcinomas, 74 yolk sac tumors, 6 choriocarcinomas, 15 mature, and 13 immature teratomas. We compared LIN28 with SALL4 to assess its diagnostic value. To determine its specificity, we examined LIN28 in 406 extragonadal-non-germ cell tumors (103 carcinomas, 91 sarcomas, 9 melanomas, 12 mesotheliomas, 83 lymphomas, 9 plasmacytomas, 82 CNS tumors, and 17 thymic epithelial tumors). The staining was semi-quantitatively scored as 0 (no cell stained), 1+ (0–30%), 2+ (31–60%), 3+ (61–90%), and 4+ (>90%). LIN28 staining was seen in all seminomas/germinomas (3+ in 1 and 4+ in 56), embryonal carcinomas (4+ in all 10), and yolk sac tumors (3+ in 3 and 4+ in 71). Variable LIN28 staining was seen in 5 of 6 choriocarcinomas (1+ to 4+), 8 of 13 immature teratomas (1+ to 2+ in immature elements), and in 1 of 15 mature teratomas (1+). Only 11 of 406 non-germ cell tumors showed 1+ LIN28 staining. Therefore, LIN28 is a sensitive (100% sensitivity) marker for primary extragonadal seminomas/germinomas, embryonal carcinomas, and yolk sac tumors with high specificity. Compared with SALL4, LIN28 demonstrated a similar level of diagnostic sensitivity for seminomas/germinomas and embryonal carcinomas. For primary extragonadal yolk sac tumors, although SALL4 stained all tumors (1+ in 1, 2+ in 2, 3+ in 10, and 4+ in 61), LIN28 stained more tumor cells (mean 95 vs 90%, P=0.03) and was therefore more sensitive. For primary extragonadal yolk sac tumors, combining LIN28 and SALL4 can achieve a higher diagnostic sensitivity than either alone.
Similar content being viewed by others
Main
LIN28, first identified in the nematode Caenorhabditis elegans, is a RNA-binding protein that has an important role in embryonic development.1 LIN28 is overexpressed in mouse and human embryonic stem cells and is important in maintaining their pluripotency.2, 3, 4 A recently study showed that in mouse, LIN28 is essential to the development of primordial germ cells and may be involved in the malignant transformation of germ cells.5 The expression of LIN28 was also found in human testicular germ cell tumors in a tissue microarray study with a limited number of cases.5 We recently explored LIN28 expression in human testicular and ovarian germ cell tumors and found that LIN28 was expressed in all primary gonadal seminomas/dysgerminomas, embryonal carcinomas, and yolk sac tumors.6, 7 LIN28 demonstrated a similar expression profile as did SALL4 in primary and metastatic gonadal germ cell tumors.6, 7 However, the status of LIN28 in primary extragonadal germ cell tumors is still unknown. Primary extragonadal germ cell tumors are believed to arise from displaced primordial germ cells and share histological, serological, and cytogenetic features with their gonadal counterparts, but they generally have a more aggressive clinical course than the latter, suggesting that there is still some difference between primary extragonadal and gonadal germ cell tumors.8, 9
This study is conducted to examine the LIN28 protein in primary extragonadal germ cell tumors using immunohistochemical staining to determine its immunohistochemical profile and to see whether LIN28 is a marker for these tumors. We also want to explore the potential value of LIN28 in diagnosing primary extragonadal germ cell tumors. Primary extragonadal germ cell tumors are uncommon tumors, but they pose more diagnostic challenges than their gonadal counterparts, and immunohistochemical markers are sometimes (probably more often than not) required to confirm their diagnosis. To achieve these two goals, we first performed immunohistochemical staining of LIN28 in 131 primary extragonadal germ cell tumors (central nervous system (CNS) 76, mediastinum 17, sacrococcygeum 30, pelvis 3, and other extragonadal sites 5). We then compared LIN28 with SALL4 for its sensitivity, a recently developed general germ cell tumor marker, in all germ cell tumors. The result of SALL4 in these germ cell tumors was previously reported.10, 11, 12 To determine its specificity, we also examined LIN28 in 82 primary non-germ cell tumors of the CNS and in 17 primary mediastinal epithelial tumors (thymomas and thymic carcinomas). The immunohistochemical status of LIN28 in other extragonadal non-germ cell tumors (103 carcinomas, 91 sarcomas, 83 lymphomas, 9 plasmacytomas, 12 mesotheliomas, and 9 melanomas) was previously reported.6, 7
Materials and methods
Case Selection
IRB approval was obtained to conduct this research. Extragonadal germ cell tumors used for this study were collected from the authors’ institutions. A total of 131 primary extragonadal germ cell tumors were collected for this study: 76 from the CNS (56 pure and 20 mixed), 17 from the mediastinum (11 pure and 6 mixed), 30 from the sacrococcygeum (18 pure and 12 with mixed), 3 from the pelvis, 2 from the vagina (both pure), 1 from the retroperitoneum (pure yolk sac tumor), 1 from the omentum (pure yolk sac tumor), and 1 from the liver (pure yolk sac tumor). The 17 primary mediastinal (thymic) epithelial tumors include 11 thymomas, 2 basaloid carcinomas, 2 lymphoepithelial-like carcinomas, 1 adenocarcinoma, and 1 poorly differentiated carcinoma. The 82 primary CNS non-germ cell tumors include 9 astrocytomas (WHO grade II 3, WHO grade III 6), 6 chordomas, 4 ependymomas (WHO grade II 2, WHO grade III 2), 11 glioblastoma multiformes, 10 medulloblastomas, 10 meningiomas (3 with chordoid features, 3 with clear cell features, 2 microcystic, and 2 fibroblastic), 5 myxopapillary ependymomas, 6 neurocytomas, 6 oligodendrogliomas (WHO grade II 3, WHO grade III 3), 8 pilocytic astrocytomas, 6 pituitary adenomas, and 1 subependymomal astrocytoma.
Immunohistochemical Staining and Evaluation
One to three formalin-fixed paraffin-embedded tissue blocks were retrieved for each case to generate 4-μm-unstained slides for immunohistochemical staining with a LIN28 polyclonal antibody (catalog no. 11724–1-AP, dilution 1:200, Proteintech Inc., Chicago, IL, USA) for all germ cell tumors and non-germ cell tumors. LIN28 immunohistochemical staining was performed on a Ventana Benchmark-XT automated stainer using the Ventana ultraView DAB detection kit. Antigen retrieval was performed using Ventana citrate-based buffer solution CC2, pH 6.0 at 95–100 °C for 24 min. LIN28 staining was cytoplasmic with some membranous accentuation. Appropriate positive and negative controls were included for each run of immunostains. The staining was scored as weak, moderate, or strong. The percentage of tumor cells labeled was semi-quantitatively scored as 0 (no tumor cells staining), 1+ (≤30% tumor cells), 2+ (31–60%), 3+ (61–90%), and 4+ (>90%).
Results
LIN28 in 76 Primary Germ Cell Tumors of the CNS
In all, 56 of 76 primary germ cell tumors were pure and 20 were mixed. They included the following tumors and tumor components: 48 germinomas, 7 embryonal carcinomas, 26 yolk sac tumors, 7 immature teratomas, 8 mature teratomas, and 5 choriocarcinomas. LIN28 staining results are summarized in Table 1.
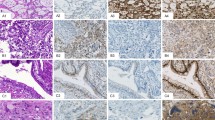
All 48 germinomas were positive for LIN28 staining, including 2+ in 2 (1 with weak staining and 1 with mixed weak and strong staining), 3+ in 1 (strong intensity), and 4+ in 45 (1 with weak staining and 44 with strong staining) (Figure 1). All 7 embryonal carcinomas showed 4+ strong LIN28 staining (Figure 1). All 26 yolk sac tumors were strongly positive for LIN28 staining, including 3+ staining in 1 (4%) and 4+ staining in 25 (96%) (Figure 2). Four of five choriocarcinomas were positive for LIN28 staining (1+ strong staining in two, 2+ staining in two including one with weak and one with strong staining) in mononucleated trophoblastic cells (Figure 3). Among syncytiotrophoblastic cells, only rare cells were weakly positive for LIN28 staining, whereas the vast majority of them were negative. Five of seven (71%) immature teratomas were positive for LIN28 staining in the immature elements (1+ in all five, three with strong staining, two with mixed weak and strong staining) (Figure 3). Most of the immature elements with LIN28 staining were immature neuroepithelium tissues, but some blastema-like immature stroma and low-grade immature mesenchyme tissues were also positive for LIN28 staining. All eight mature teratomas were negative for LIN28 staining.
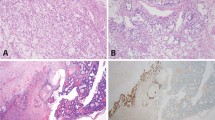
Primary extragonadal yolk sac tumors also display multiple histological patterns (a1, Schiller–Duval body; b2, solid and parietal; c1, microcystic). All primary extragonadal yolk sac tumors showed positive SALL4 staining (a2–d2), with strong staining in >90% tumor cells in 61 of 74 (82%) cases (a2–c2), but some (13/74) primary extragonadal yolk sac tumors showed SALL4 staining in <90% tumor cells including one in the central nervous system (d1) in which only a very small percentage of tumor cells were positive for SALL4 staining (d2) (this tumor is also positive for α-fetoprotein and glypican-3 in 80 and 70% tumor cells, respectively). Compared with SALL4, LIN28 demonstrated strong staining in >90% tumor cells in 71 of 74 (96%) cases (a3–d3) and in 75–85% cells in 3 cases. Among the 13 cases showing SALL4 staining in <90% tumor cells (d2), 12 showed LIN28 staining in >90% tumor cells (d3).
LIN28 in 17 Primary Mediastinal Germ Cell Tumors
Among the 17 primary mediastinal germ cell tumors, 11 were pure and 6 were mixed. The tumors contained the following tumors and tumor components: 9 seminomas (6 pure and 3 as a component in mixed germ cell tumors), 10 yolk sac tumors (4 pure and 6 as a component in mixed germ cell tumors), 3 embryonal carcinomas (all as a component in mixed germ cell tumors), 1 choriocarcinoma (in a mixed germ cell tumor), 2 mature teratomas (both as a component in mixed germ cell tumors), and 3 immature teratomas (1 pure and 2 as a component in mixed germ cell tumors).
Strong 4+ LIN28 staining was seen all seminomas (9/9, 100%), embryonal carcinomas (3/3, 100%), and yolk sac tumors (10/10, 100%) (Table 2). LIN28 staining was negative in all mature and immature teratomas. (Table 2) The only choriocarcinomatous component showed 4+ weak LIN28 staining (the amount of choriocarcinomatous component was small: almost all mononucleated trophoblastic cells and one of two syncytiotrophoblastic cells was weakly positive) (Table 2).
LIN28 in 30 Primary Sacrococcygeal and 3 Primary Pelvic Germ Cell Tumors
The tumors in these 30 patients with sacrococcygeal germ cell tumors were pure yolk sac tumors in 18 (60%) and mixed yolk sac tumors and teratomas in 12 (40%) (7 immature and 5 mature) patients. The three patients with pelvic germ cell tumors had pure yolk sac tumors. All 33 yolk sac tumors showed strong LIN28 staining, including 4+ in 31 (94%) and 3+ in 2 (6%) (75 and 85% cells, respectively) (Table 3). In all, 8 of 12 cases with mixed teratomas and YSTs had teratoma present on the sections stained for LIN28 (3 immature and 5 mature) (Table 3). Positive LIN28 was seen in all three immature teratomas (1+ weak to strong in two and 2+ moderate to strong in one) with the staining pattern similar to that observed in immature teratomas of the CNS, and in one of five mature teratomas (5% or 1+ weak staining in the teratomatous glands) (Table 3).
LIN28 in Five Primary Extragonadal Germ Cell Tumors of Other Sites
Five extragonadal germ cell tumors were from sites other than the CNS, mediastinum, sacrococcygeum, or pelvis. Two of these tumors were from the vagina (7- and 22-month-old females, respectively), one from the retroperitoneum (2-year-old female), and one from the liver (7-month-old female). All were pure yolk sac tumors and all showed strong 4+ LIN28 staining (Table 3).
LIN28 in Primary Non-Germ Cell Tumors of the CNS and Mediastinum
Only 1 of the 82 primary non-germ cell tumors of the CNS was positive for LIN28 staining (1% tumor cells with weak staining in 1 medulloblastoma). None of the 17 thymic epithelial neoplasms showed LIN28 staining.
Comparison of LIN28 and SALL4 in Extragonadal Germ Cell Tumors
The comparison between LIN28 and SALL4 in these extragonadal germ cell tumors was summarized in Table 4. The results of SALL4 in these germ cell tumors were previously reported.10, 11, 12
All 57 seminomas/germinomas showed positive staining for both LIN28 (2+ in 2, 3+ in 1, and 4+ in 54) and SALL4 (3+ in 1 and 4+ in 56). Strong 4+ staining was seen in all 10 ECs for both LIN28 and SALL4. All 74 yolk sac tumors were positive for both LIN28 (3+ in 3 and 4+ in 71) and SALL4 (1+ in 1, 2+ in 2, 3+ in 10, and 4+ in 61). In all, 61 of 74 (82%) yolk sac tumors showed strong 4+ staining for both LIN28 and SALL4. Among the 3 YSTs with 3+ LIN28 staining (75, 85, and 85%, respectively), 2+ showed 4+ SALL4 staining and 1 showed 3+ SALL4 staining. Among the 13 yolk sac tumors with 1+ to 3+ SALL4 staining, 12 showed 4+ LIN28 staining and 1 showed 3+ LIN28 staining. Taken together, 73 of 74 yolk sac tumors showed 4+ staining (>90% tumor cells) with LIN28 and/or SALL4. The remaining yolk sac tumor showed 3+ staining for both LIN28 and SALL4. In all 74 yolk sac tumors, the mean percentage of tumor cells stained was 95% for LIN28 and 90% for SALL4 (P=0.03).
Three of six choriocarcinomas were positive for both LIN28 and SALL4 (20 vs 40% cells, 50 vs 25%, 30 vs 10%, respectively). Two additional choriocarcinomas were positive for LIN28 (50 and 90% cells, respectively) but negative for SALL4. The remaining one choriocarcinoma was positive for SALL4 staining (1–2% cells) but negative for LIN28 staining. Among the 13 immature teratomas, 8 were positive for LIN28 staining and all these 8 were also positive for SALL4 (7 with 1+ staining for both, the remaining 1 case with 2+ LIN28 and 1+ SALL4 staining). No immature teratoma in this study was positive only for LIN28, and not for SALL4 or vice versa. Only 1 of 15 mature teratomas showed 1+ SALL4 staining in the teratomatous glands. Another mature teratoma was weakly positive for LIN28 staining (5% cells in the teratomatous glands).
Discussion
In this study, we performed immunohistochemical staining of LIN28 in 131 primary extragonadal germ cell tumors. Our results showed that positive LIN28 staining was seen in all 56 seminomas/germinomas, 10 embryonal carcinomas, and all 74 yolk sac tumors, as well as some teratomas and choriocarcinomas. The expression profile of LIN28 in these primary extragonadal germ cell tumors indicates that LIN28 is a highly sensitive (100% sensitivity) marker for primary extragonadal seminoma/dysgerminoma, embryonal carcinoma, and yolk sac tumor. For these tumors, strong LIN28 staining in >90% tumor cells was seen in 53 of 57 (93%), 10 of 10 (100%), and 71 of 74 (96.0%) tumors, respectively.
LIN28 is not only sensitive but also highly specific for primary extragonadal seminoma/germinoma, embryonal carcinoma, and yolk sac tumor. In this study, among the 82 primary non-germ cell tumors of the CNS, only 1 medulloblastoma showed weak staining in 1% tumor cells. None of the 17 thymic epithelial neoplasms showed LIN28 staining. In our recent studies, among other types of 307 extragonadal non-germ cell tumors (103 metastatic carcinomas of major types, 91 sarcomas of various types, 9 metastatic melanomas, 12 mesotheliomas, 83 lymphomas of various types, and 9 plasmacytomas), only 10 (6 carcinomas, 1 sarcoma, and 3 melanomas) showed weak LIN28 staining in no >30% tumor cells.6, 7 Although further studies to include more extragonadal non-germ cell tumors are required, our findings do indicate that LIN28 is a highly specific marker for primary seminomas/germinomas, embryonal carcinomas, and yolk sac tumors in extragonadal sites. Using 4+ staining as the criteria, LIN28 is 100% specific for seminomas/germinomas, embryonal carcinomas, and yolk sac tumors (except rare choriocarcinoma may show strong 4+ LIN28 staining).
The high sensitivity of LIN28 with the relatively high specificity for primary extragonadal seminomas/dysgerminomas, embryonal carcinomas, and yolk sac tumors indicate that LIN28 can be used as a diagnostic marker for them. Primary extragonadal germ cell tumors are uncommon tumors that most often occur in the midline structures and mediastinum, but they can also occur in many other organs, not only in children but also in adults.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Morphologically, primary extragonadal germ cell tumors can mimic many types of non-germ cell tumors. For example, mediastinal seminomas may mimic type B thymomas and embryonal carcinoma may mimic other types of carcinomas. The yolk sac tumor is notorious for displaying multiple histological patterns and can mimic adenocarcinoma, myxopapillary ependymoma, and chordoma, etc. The diagnosis of primary extragonadal germ cell tumors can be difficult without immunohistochemical staining. Recently, stem cell markers such as SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 have emerged as more sensitive and specific markers for germ cell tumors than non-stem cell markers, such as C-KIT, α-fetoprotein, placental-like alkaline phosphatase, CD30, and glypican-3.10, 11, 12, 27, 28, 29, 30, 31, 32, 33, 34 Among these stem cell markers, SALL4 has demonstrated the broadest expression profile and is the only one to label yolk sac tumor.10, 11, 12 We compared LIN28 with SALL4 for its diagnostic utility in all primary extragonadal germ cell tumors. Both LIN28 and SALL4 showed 100% sensitivity for seminomas/germinomas and embryonal carcinomas with staining in >90% tumor cells in nearly all or all cases (95 and 98% for seminomas/germinomas, 100 and 100% for embryonal carcinomas, respectively), indicating that LIN28 had a similar level of diagnostic sensitivity as did SALL4 for primary extragonadal seminomas/germinomas and embryonal carcinomas. LIN28 staining is cytoplasmic, whereas for SALL4, it is nuclear. However, both LIN28 and SALL4 have a clean staining background. In our experience, the reading of LIN28 is as easy as the reading of SALL4.
For primary extragonadal yolk sac tumors, LIN28 demonstrated a higher sensitivity than did SALL4. The yolk sac tumor is the extragonadal germ cell tumor that poses the greatest diagnostic challenges because of its multiple histological patterns and wide organ distribution.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Previous markers for diagnosing extragonadal yolk sac tumors included placental-like alkaline phosphatase, α-fetoprotein, and glypican-3, but they lack adequate sensitivity and specificity. Recent studies have shown that SALL4 is a more sensitive marker for primary extragonadal yolk sac tumors.10, 11, 12 However, unlike gonadal yolk sac tumors, some (13/74 or 18%) primary extragonadal YSTs showed SALL4 staining in a non-diffuse (<90% cells) pattern (SALL4 staining in <30% cells in 1 CNS yolk sac tumor, in between 30 and 60% cells in 2 tumors (1 in the CNS and 1 in the mediastinum), and in between 60 and 90% cells in 10 tumors (7 in the CNS and 3 in other sites)),10, 11, 12 and such a staining pattern may limit the diagnostic utility of SALL4 in certain situations, given the fact that many specimens obtained from the CNS and mediastinum are small biopsies. In small biopsies, a negative SALL4 staining does not necessarily rule out a yolk sac tumor. Therefore, it will be helpful to identify another sensitive marker for extragonadal yolk sac tumors to complement SALL4 to further increase the diagnostic sensitivity for extragonadal yolk sac tumor. Although all these 13 yolk sac tumors with 1+ to 3+ SALL4 staining were also positive for α-fetoprotein and glypican-3, 11 and 10 showed staining for α-fetoprotein and glypican-3 in fewer tumor cells than for SALL4, respectively.10, 11, 12 In our study, 71 of 74 extragonadal yolk sac tumors had LIN28 staining in >90% tumor cells (4+), and only 3 showed staining in 60–90% tumor cells (3+). Among 13 extragonadal yolk sac tumors with 1+ to 3+ SALL4 staining, 12 showed 4+ LIN28 staining and 1 showed 3+ LIN28 staining. Although SALL4 also stained all primary extragonadal yolk sac tumors, the mean percentage of tumor cells stained was higher for LIN28 staining than for SALL4 (95 vs 90%, P=0.03). Therefore, LIN28 is a more sensitive marker for primary extragonadal yolk sac tumor than is SALL4 and can further improve the diagnostic sensitivity for these tumors. With a combination of SALL4 and LIN28, we can achieve a greater sensitivity for extragonadal yolk sac tumors, even in small biopsies, than with LIN28 or SALL4 alone. In our study, 73 of 74 (99%) extragonadal yolk sac tumors showed staining in >90% tumor cells for either SALL4 and/or LIN28.
In previous studies, we explored the immunohistochemical profile of LIN28 in a large series of primary and metastatic gonadal germ cell tumors (184 testicular and 79 ovarian).6, 7 When compared with primary gonadal germ cell tumors, primary extragonadal germ cell tumors showed a similar profile of LIN28 in each subtype of tumor. The finding of LIN28 expression in primary extragonadal germ cell tumors may help understand their pathogenesis. LIN28 has been shown to be essential to the development of primordial germ cell development in mouse embryo and is also important in maintaining the growth of mouse embryonic carcinoma cells and the primitive nature of the immature teratoma in immunodeficient mice.5 Primary extragonadal germ cell tumors are believed to arise from germ cells, which are most likely displaced in the extragonadal sites because of aberrant migration during development.8 On the basis of gene imprinting, cytogenetic, and immunohistochemical studies, it has been proposed that extragonadal germ cell tumors might evolve through the same pathogenetic pathways as primary gonadal germ cell tumors do.8, 9 A similar expression profile of LIN28 between primary extragonadal and gonadal germ cell tumors provides further indirect evidence to support such a hypothesis.
Finally, it must be pointed out that in extragonadal sites, diffuse and strong LIN28 staining in a tumor highly indicates a malignant germ cell tumor but it does not distinguish a primary extragonadal germ cell tumor from a metastatic one (from gonad or another extragonadal site) because metastatic gonadal germ cell tumors to extragonadal sites and primary extragonadal germ cell tumors had identical LIN28 profile in each subtype.6, 7 In this situation, clinical information is required to determine whether an extragonadal germ cell tumor is primary or metastastic.
In summary, we performed immunohistochemical staining of LIN28 in 131 primary extragonadal germ cell tumors. Our results showed that LIN28 is a highly sensitive marker for primary extragonadal seminomas/germinomas, embryonal carcinomas, and yolk sac tumors with high specificity. Variable LIN28 expression was also seen in some teratomas and choriocarcinomas. When compared with SALL4, LIN28 demonstrated a similar expression profile in these primary extragonadal germ cell tumors. LIN28 can be used as a diagnostic marker for primary extragonadal seminomas/germinomas, embryonal carcinomas, and yolk sac tumors. It showed a similar level of diagnostic sensitivity as SALL4 for primary extragonadal seminomas/germinomas and embryonal carcinomas. LIN28 is more sensitive than SALL4 for primary extragonadal yolk sac tumors. Combining LIN28 and SALL4 can further increase the diagnostic sensitivity for primary extragonadal yolk sac tumors.
References
Ambros V, Horvitz HR . Heterochronic mutants of the nematode Caenorhabditis elegans. Science 1984;226:409–416.
Viswanathan SR, Daley GQ . Lin28: a microRNA regulator with a macro role. Cell 2010;140:445–449.
Richards M, Tan SP, Tan JH, et al. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells 2004;22:51–64.
Qiu C, Ma Y, Wang J, et al. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res 2010;38:1240–1248.
West JA, Viswanathan SR, Yabuuchi A, et al. A role for Lin28 in primordial germ-cell development and germ cell malignancy. Nature 2009;460:909–913.
Cao D, Allan RW, Cheng L, et al. RNA-binding protein LIN28 is a marker for testicular germ cell tumors. Hum Pathol (in press).
Xue D, Peng Y, Wang F, et al. Diagnostic utility of LIN28 in ovarian primitive germ cell tumors. Histopathology (in press).
Oosterhuis JW, Stoop H, Honecker F, et al. Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. Int J Androl 2007;30:256–263.
Schmoll HJ . Extragonadal germ cell tumors. Ann Oncol 2002;13 (Suppl 4):265–272.
Mei K, Liu A, Allan RW, et al. Diagnostic utility of SALL4 in primary germ cell tumors of the central nervous system: a study of 77 cases. Mod Pathol 2009;22:1628–1636.
Liu A, Cheng L, Du J, et al. Diagnostic utility of novel stem cell markers SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 in primary mediastinal germ cell tumors. Am J Surg Pathol 2010;34:697–706.
Wang F, Liu A, Peng Y, et al. Diagnostic utility of SALL4 in extragonadal yolk sac tumors: an immunohistochemical study of 59 cases with comparison to placental-like alkaline phosphatase, alpha-fetoprotein, and glypican-3. Am J Surg Pathol 2009;33:1529–1539.
Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol 2002;20:1864–1873.
Moran CA, Suster S, Przygodzki RM, et al. Primary germ cell tumors of the mediastinum: II. Mediastinal seminomas—a clinicopathologic and immunohistochemical study of 120 cases. Cancer 1997;80:691–698.
Moran CA, Suster S, Koss MN . Primary germ cell tumors of the mediastinum: III. Yolk sac tumor, embryonal carcinoma, choriocarcinoma, and combined nonteratomatous germ cell tumors of the mediastinum—a clinicopathologic and immunohistochemical study of 64 cases. Cancer 1997;80:699–707.
Goss PE, Schwertfeger L, Blackstein ME, et al. Extragonadal germ cell tumors. A 14-year Toronto experience. Cancer 1994;73:1971–1979.
Iczkowski KA, Butler SL, Shanks JH, et al. Trials of new germ cell immunohistochemical stains in 93 extragonadal and metastatic germ cell tumors. Hum Pathol 2008;39:275–281.
Hsu YJ, Pai L, Chen YC, et al. Extragonadal germ cell tumors in Taiwan: an analysis of treatment results of 59 patients. Cancer 2002;95:766–774.
Clement PB, Young RH, Scully RE . Extraovarian pelvic yolk sac tumors. Cancer 1988;62:620–626.
Basgul A, Gokaslan H, Kavak ZN, et al. Primary yolk sac tumor (endodermal sinus tumor) of the vulva: case report and review of the literature. Eur J Gynaecol Oncol 2006;27:395–398.
Geminiani ML, Panetta A, Pajetta V, et al. Endodermal sinus tumor of the omentum: case report. Tumori 2005;91:563–566.
Gunawardena SA, Siriwardana HP, Wickramasinghe SY, et al. Primary endodermal sinus (yolk sac) tumour of the liver. Eur J Surg Oncol 2002;28:90–91.
Warren M, Thompson KS . Two cases of primary yolk sac tumor of the liver in childhood: case reports and literature review. Pediatr Dev Pathol 2009;12:410–416.
Wong NA, D’Costa H, Barry RE, et al. Primary yolk sac tumour of the liver in adulthood. J Clin Pathol 1998;51:939–940.
Terenziani M, Spreafico F, Collini P, et al. Endodermal sinus tumor of the vagina. Pediatr Blood Cancer 2007;48:577–578.
Lack EE . Extragonadal germ cell tumors of the head and neck region: review of 16 cases. Hum Pathol 1985;16:56–64.
Jones TD, Ulbright TM, Eble JN, et al. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol 2004;28:935–940.
Santagata S, Ligon KL, Hornick JL . Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol 2007;31:836–845.
Cao D, Li J, Guo CC, et al. SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am J Surg Pathol 2009;33:1065–1077.
Cao D, Humphrey PA, Allan RW . SALL4 is a novel sensitive and specific marker for metastatic germ cell tumors, with particular utility in detection of metastatic yolk sac tumors. Cancer 2009;115:2640–2651.
Wang P, Li J, Allan RW, et al. Expression of UTF1 in primary and metastatic testicular germ cell tumors. Am J Clin Pathol 2010;134:604–612.
Santagata S, Hornick JL, Ligon KL . Comparative analysis of germ cell transcription factors in CNS germinoma reveals diagnostic utility of NANOG. Am J Surg Pathol 2006;30:1613–1618.
Cao D, Lane Z, Allan RW, et al. TCL1 is a novel diagnostic marker for intratubular germ cell neoplasia and classic seminoma. Histopathology 2010;57:152–157.
Lau SK, Weiss LM, Chu PG . TCL1 protein expression in testicular germ cell tumors. Am J Clin Pathol 2010;133:762–766.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cao, D., Liu, A., Wang, F. et al. RNA-binding protein LIN28 is a marker for primary extragonadal germ cell tumors: an immunohistochemical study of 131 cases. Mod Pathol 24, 288–296 (2011). https://doi.org/10.1038/modpathol.2010.195
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.195
Keywords
This article is cited by
-
Functional and clinical significance of SALL4 in breast cancer
Tumor Biology (2016)