Abstract
Thyroid carcinomas can be sporadic or familial. Familial syndromes are classified into familial medullary thyroid carcinoma (FMTC), derived from calcitonin-producing C cells, and familial non-medullary thyroid carcinoma, derived from follicular cells. The familial form of medullary thyroid carcinoma (MTC) is usually a component of multiple endocrine neoplasia (MEN) IIA or IIB, or presents as pure FMTC syndrome. The histopathological features of tumors in patients with MEN syndromes are similar to those of sporadic tumors, with the exception of bilaterality and multiplicity of tumors. The genetic events in the familial C-cell-derived tumors are well known, and genotype–phenotype correlations well established. In contrast, the case for a familial predisposition of non-medullary thyroid carcinoma is only now beginning to emerge. Although, the majority of papillary and follicular thyroid carcinomas are sporadic, the familial forms are rare and can be divided into two groups. The first includes familial syndromes characterized by a predominance of non-thyroidal tumors, such as familial adenomatous polyposis and PTEN-hamartoma tumor syndrome, within others. The second group includes familial syndromes characterized by predominance of papillary thyroid carcinoma (PTC), such as pure familial PTC (fPTC), fPTC associated with papillary renal cell carcinoma, and fPTC with multinodular goiter. Some characteristic morphologic findings should alert the pathologist of a possible familial cancer syndrome, which may lead to further molecular genetics evaluation.
Similar content being viewed by others
Main
Medullary thyroid carcinoma (MTC) is a rare C-cell calcitonin-producing tumor, and occurs in sporadic and familial forms. The familial form of MTC accounts for 20–25% of cases, and is usually a component of multiple endocrine neoplasia (MEN) IIA or IIB, or presents as pure familial MTC (FMTC) syndrome. The genetic events in the familial C-cell-derived tumors are well known and genotype–phenotype correlations are well established. In contrast, the case for a familial predisposition of non-medullary thyroid carcinoma (NMTC) is only now beginning to emerge.
Although the majority of papillary (papillary thyroid carcinoma, PTC) and follicular thyroid carcinomas (FTCs) are sporadic,1, 2, 3, 4, 5, 6, 7 familial tumors may account for 5–15% of thyroid carcinoma cases. The presence of multifocal papillary carcinoma is a common feature of familial NMTC (FNMTC).
The familial follicular cell-derived tumors or NMTCs encompass a heterogeneous group of diseases, including both syndromic-associated tumors and non-syndromic tumors based on clinico-pathologic findings.1, 2 The first group (syndromic associated) has an increased prevalence of NMTC within a familial cancer syndrome with a preponderance of non-thyroidal tumors (familial tumor syndromes characterized by a preponderance of non-thyroidal tumors). Thyroid neoplasia has been reported with increased frequency in familial syndromes, such as familial adenomatous polyposis (FAP), PTEN-hamartoma tumor syndrome (PHTS), Carney's complex type 1, and Werner's syndrome. Thyroid carcinomas in multitumor genetic syndromes are heterogeneous diseases, tend to share some similar characteristics including early age onset, and are usually bilateral and multicentric.
In the second group (non-syndromic tumors), the predominant neoplasm is NMTC (familial tumor syndromes characterized by a predominance of NMTC; FNMTC), although other neoplasms may occur with increased frequency. This second group also includes familial syndromes characterized by a predominance of NMTC, such as pure familial (f) PTC with or without oxyphilia, fPTC with papillary renal cell carcinoma, and fPTC with multinodular goiter. FNMTC is characterized by three or more first-degree relatives with follicular-derived NMTC, and occurs regardless of the presence of another familial syndrome.
The pathologist has a critical function in the identification of these syndromes by recognizing characteristic patterns of thyroid morphologic findings, and often involves interaction between pathologists and surgeons, which may lead to further molecular genetics evaluation.1, 2
Familial medullary thyroid carcinoma
MTC refers to those neoplasms arising from the calcitonin-producing C thyroid cells derived from neural crest, and represents approximately 5% of all thyroid tumors, and about 15% of all thyroid cancer-related deaths. In 1966, Williams identified the histogenesis of this tumor as C-cell origin.7
MTCs occur in sporadic or hereditary (25% of cases) forms, as part of MEN II syndromes or as the MTC-only syndrome.8 MEN II syndrome consist of three variants, MEN IIA, MEN IIB, and FMTC. MEN IIA is associated with pheochromocytoma and parathyroid hyperplasia, whereas MEN IIB is associated with marfanoid habitus, mucosal neuromas, ganglioneuromatosis, and pheochromocytoma (Table 1).
MTC can occur in the four different settings:
Sporadic
Sporadic accounts up to 80% of all cases of medullary thyroid cancer. They are typically unilateral and there are no associated endocrinopathies (not associated with disease in other endocrine glands). Peak of onset is between 40 and 60 years of age, with a mean age 50 years. The tumors are unilateral. Females outnumber males by 3:2 ratio. One-third will present with intractable diarrhea. Diarrhea is caused by increased gastrointestinal secretion and hypermotility because of the hormones secreted by the tumor (calcitonin, prostaglandins, serotonin, or VIP).
FMTC or Inherited Medullary Carcinoma without Associated Endocrinopathies
This form of medullary carcinoma is the least aggressive. This group of MTC patients usually develops no other clinical manifestations. Similar to other types of thyroid cancers, the peak incidence is between the ages of 40 and 50 years.
MEN IIA (Sipple Syndrome)
MEN IIA syndrome or Sipple syndrome has bilateral medullary carcinoma or C-cell hyperplasia (CCH), pheochromocytoma, and hyperparathyroidism. This syndrome is inherited as an autosomal dominant manner, and males and females are equally affected. Peak incidence of medullary carcinoma in these patients is in the 30s, as late adolescence or early adulthood.
MEN IIB
MEN IIB is associated with pheochromocytoma, mucosal ganglioneuromas, and marfanoid habitus. This syndrome also has medullary carcinoma and pheochromocytoma, but only rarely will have hyperparathyroidism. Instead, these patients have an unusual appearance, which is characterized by mucosal ganglioneuromas and a marfanoid habitus. Inheritance is autosomal dominant as in MEN IIA. MEN IIB patients usually get medullary carcinoma early in life, diagnosed in infancy or early childhood before their 30s, and males and females are equally affected.
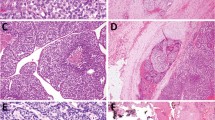
Both sporadic and FMTC arise at the junctions of the upper and middle thirds of the lateral lobes, corresponding to the areas where C cells are present. The terminology of CCH refers to two different pathological conditions, referred as neoplastic CCH and reactive CCH. Reactive CCH is seen in association with hyperparathyroidism, lymphocytic thyroiditis, aging, and adjacent to a follicular tumor. Neoplastic CCH or MTC in situ is the precursor lesion of heritable MTC.9 The hereditary MTC is usually preceded by CCH, and usually occurs in the upper two-thirds of the thyroid adjacent to an MTC or seen in prophylactic thyroidectomy of asymptomatic carriers of RET mutation (Figure 1). Multiple CCH is a hallmark of MEN II syndromes and FMTC syndrome. The presence of neoplastic CCH is considered a paradigm of a genetically determined condition.9, 10
Sporadic MTC tumors are typically unilateral, and grossly they are white-gray-yellow, firm, and gritty (Figure 2). The histopathology is quite variable and numerous histological subtypes are described.7
MTC in association with MEN II syndromes and in FMTC patients are usually bilateral, multicentric, and well demarcated (Figure 3). Although the tumors are sharply circumscribed, they are not encapsulated. MTC typically is the first abnormality observed in both MEN IIA and MEN IIB syndromes. The characteristic histopathological features of MTC in both sporadic and familial are sheets, nests, or trabeculae of round or polygonal or spindle or giant cells with a variable amount of fibrous or amyloid stroma (Figure 4). However, at least 12 variants are described, including glandular, plasma cell-like, giant cell, spindle cell, papillary, small cell, paraganglioma-like, oncocytic cell, clear cell, angiosarcoma-like, squamous cell, melanin –producing, and amphicrine.7
In summary, thyroid pathology in FMTC cases usually show
-
multiple tumors
-
bilateral tumors
-
associated with neoplastic CCH
-
show early lymph node metastases.
A germline point mutation in the RET gene on chromosome 10q11.2 is responsible for the hereditary MTC. The RET protooncogene has 21 exons distributed over 60 kb. Analysis of the nucleotide sequence revealed that it encodes a receptor tyrosine kinase with four cadherin-related repeats and a cysteine-rich region in the extracellular domain (Figure 5). About 85% of all mutations responsible for FMTC are well known. In the majority of MEN IIA and FMTC patients, mutations are clustered in six cysteine residues (codons 609, 611, 618, and 620 in exon 10, and codons 630 and 634 in exon 11) in the RET cysteine-rich extracellular domain (Figure 5). These mutations have been detected in about 95% of MEN IIA and 85% of FMTC families. Somatic RET point mutations have been identified in about 50% of patients with sporadic MTC.11
The aggressiveness of MTC is usually related to the clinical presentation, whether presents as a hereditary or sporadic forms, and the type of RET mutation present.12, 13, 14, 15, 16, 17, 18, 19 Sporadic MTC can present at any age, and it is usually associated with a palpable mass and the presence of nodal metastases. In familial syndromes, there is a known relationship between specific RET protooncogene mutations, disease phenotype, and prognosis. The aggressiveness and age of onset of FMTC differs depending on the specific genetic mutation, and this should determine the timing and extent of surgery.
All patients with MTC should then be screened for familial disease. Genetic testing is considered the standard of care for all first-degree relatives of patients with newly diagnosed MTC. MEN II provides a unique model for early prevention and cure of cancer.12, 13, 14, 15, 16, 17, 18, 19
According to the American Thyroid Association, preoperative laboratory testing in patients with suspected MTC has three purposes:
-
1
Predict the extent of metastatic disease; this will determine the extent of preoperative imaging and may alter the surgical approach.
-
2
In patients with MEN II, identify primary hyperparathyroidism and/or pheochromocytoma; these conditions may alter the surgical approach and surgical priorities.
-
3
Identify RET mutation carriers so that testing of appropriate family members can allow for early diagnosis and treatment of affected individuals.
Prophylactic thyroidectomies are increasingly performed on patients at risk for developing MTC, performed usually in patients with family history of MEN or MTC, elevated serum calcitonin level, or detection of an RET protooncogene mutation (Table 2). The consensus in the literature is that the thyroids in these patients submitted to thyroidectomies are associated with CCH and/or medullary microcarcinomas.20, 21, 22
Familial non-medullary thyroid carcinoma
Familial forms of follicular-derived neoplasms have been only acknowledged in recent years. Presently, approximately 5–15% of non-medullary thyroid cancers are considered to be of familial origin. The familial follicular cell-derived tumors or FNMTC encompass a heterogeneous group of diseases, including both syndromic-associated tumors and non-syndromic tumors1, 2 (Table 3).
The first group has an increased prevalence of NMTC within a familial cancer syndrome with a preponderance of non-thyroidal tumors (familial tumor syndromes characterized by a preponderance of non-thyroidal tumors).
NMTC has been found with a greater frequency than expected in Cowden's syndrome (CS) (PHTS), FAP, Carney's complex, and Werner's syndrome, within others. Thyroid carcinomas in multitumor genetic syndromes are heterogeneous diseases, and tend to share some similar characteristics including early age onset, multicentricity, and bilaterality (Table 4). In FAP, there is a marked female preponderance under the age of 30 years. Papillary carcinoma is the most common type and affects only 2% of patients. In another familial syndrome, PHTS, thyroid neoplasia is the most frequent extracutaneous manifestation. In this syndrome, over 65% of patients have thyroid disease, including numerous adenomatous nodules, lymphocytic thyroiditis, follicular adenomas, and carcinomas, and less frequently PTCs. The thyroid pathology of these two syndromes will be discussed in detail below. Other familial syndromes associated with thyroid neoplasia include Carney's complex and Werner's syndrome (Table 5).
In the second group, the predominant neoplasm is NMTC (familial tumor syndromes characterized by a predominance of NMTC; FNMTC), although other neoplasms may occur with increased frequency. Non-syndromic or familial tumor syndromes characterized by a predominance of NMTC are sub classified in different groups as pure fPTC with or without oxyphilia, fPTC with renal papillary tumor, and fPTC with multinodular goiter (Table 6). FNMTC is characterized by three or more first-degree relatives with follicular-derived NMTC, and occurs regardless of the presence of another familial syndrome.
The presence of multifocal, multinodular, and bilateral thyroid neoplasms is suggestive of a familial disease. The characteristic morphological findings should alert the pathologist to the possibility of a familial cancer syndrome,1 as the correct histological interpretation may lead to further molecular genetic evaluation of the patient and family members.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37
Familial Tumor Syndromes Characterized by a Preponderance of Non-thyroidal Tumors
Familial adenomatous polyposis
FAP is an inherited autosomal dominant syndrome caused by germline mutations in the adenomatous polyposis coli (APC) gene on chromosome 5q21, characterized by hundreds of adenomatous colonic polyps that develop during early adulthood. Extracolonic manifestations in FAP include osteomas, epidermal cysts, desmoid tumors, upper gastrointestinal tract polyps hamartomas, congenital hypertrophy of the retinal pigmented epithelium (CHRPE), hepatoblastomas, and thyroid tumors. PTC is one of the extracolonic manifestations of FAP, and occurs in approximately 2% of patients.38, 39, 40 Young women with FAP are at particular risk of developing thyroid cancer, and their chance of being affected is approximately 160 times higher than that of normal individuals; PTC occurs with a frequency of about 10 times greater than that expected for sporadic PTC.
Thyroid carcinomas associated with FAP are usually bilateral and multifocal. The histological features are different from sporadic tumors38 with the characteristic cribriform pattern having solid areas and a spindle cell component, most often associated with marked fibrosis. The cribriform–morular variant of PTC (CMv–PTC) described originally as FAP-associated thyroid carcinoma is a very rare subtype of PTC representing approximately 0.1–0.2% of all papillary carcinoma cases. Among patients with FAP who have synchronous PTC, over 90% of these cases have been reported to exhibit histologic features of the CMv. The characteristic cellular and nuclear findings of sporadic PTC, such as nuclear grooving, overlapping, intranuclear inclusions, and clear nuclei are rare to absent in this subtype.38, 39, 40
The CMv of thyroid carcinoma (CMv–TC) typically occurs as an extraintestinal manifestation of FAP, although rare sporadic cases have been reported. It occurs almost exclusively in young females, is well differentiated, often multifocal, is characterized by cribriform, solid, and morular areas lacking typical nuclear features of PTC (Figure 6), and is associated with germline and somatic mutations in the APC and β-catenin genes. In contrast to conventional PTC, CMv–TC rarely metastasizes and carries a benign prognosis. CMv–TC is characterized by aberrant nuclear and cytoplasmic expression of β-catenin (β-catenin immunostaining is strong in cytoplasm and nuclei in the morular and cribriform areas, and it is only expressed in cell membrane of the non-tumoral follicular cells) (Figure 6); immunoreactivity for CK19, p53, and Bcl-2; and lack of immunoreactivity for HBME-1 and galectin-3; these features are distinct from variants of PTC, including classical, tall cell, and diffuse sclerosing variants.
The overall prognosis of the CMv–PTC is similar to that of classical variant of PTC with <10% of cases showing an aggressive clinical behavior. The distinct CMv–PTC seen in FAP-related thyroid carcinomas is very unusual in sporadic PTC, and its identification should raise the possibility of this familial tumor syndrome. Any patient presenting with this rare subtype of papillary carcinoma should be evaluated for FAP. A recent study41 showed a 12% prevalence of thyroid cancer in FAP, which is significantly higher than previously reported (2%), and recommended close follow-up ultrasound screening. Thyroid carcinomas associated with FAP show a very good prognosis, with rare metastases or poor outcome reported in most series with a long-term follow-up.38, 39, 40, 41, 42, 43
The APC gene is ubiquitously expressed in normal tissue, and it is a negative regulator of Wnt pathway. Inactivation of the APC tumor suppressor gene initiates colorectal neoplasia, and is also involved in FAP-related thyroid tumors. Mutations of the APC gene lead to a truncated protein that lacks the β-catenin binding site and, therefore, cannot degrade β-catenin. One of the biochemical activities associated with the APC protein is down-regulation of transcriptional activation mediated by β-catenin. Colorectal tumors with intact APC genes were found to contain activating mutations of β-catenin that altered functionally significant phosphorylation sites. These results indicate that regulation of β-catenin is critical to APC tumor suppressive effect. The molecular genetic findings in patients affected by papillary carcinoma43 concluded that 21 of 24 (87.5%) germline mutations of APC gene were in exon 15 in the genomic area associated with CHRPE (codons 463–1387), and 22 of 24 (92%) germline mutations were out of the mutation cluster region (codons 1286–1513), currently considered the hot spot mutation area. Uchino et al44 found no mutation of the β-catenin gene on the thyroid (12 CMv–PTC) specimen or in peripheral blood of an FAP patient, although R554X germline mutation and six different somatic mutations were identified on the APC gene. Somatic mutation in exon 3 of β-catenin was reported in two patients with FAP-associated thyroid carcinoma and in three cases considered ‘sporadic’ CMv–PTC.45 Cetta et al43 recommended intensive screening for thyroid nodules after the age of 15 years if a single patient or entire kindred have CHRPE and/or mutations in the 5′-portion of exon 15. Somatic mutation of the APC gene has been reported in FAP thyroid tumors.39, 40, 41, 42, 43, 44
We suggest an algorithmic approach for a diagnostic and management of enlarged and multinodular thyroid with multifocal CMv–PTC (Figure 7).
PTEN-hamartoma tumor syndrome
PHTS is a complex disorder caused by germline inactivating mutations of the PTEN tumor suppressor gene, which maps to 10q23.3, and includes CS and Bannayan–Riley–Ruvalcaba syndrome (BRRS), within others. In CS, germline intragenic PTEN mutations have been found in up to 80–85% of classically affected patients. For patients with a clinical diagnosis of BRRS, intragenic mutations account for 60% of the cases. Over 90% of individuals affected with CS manifest a phenotype by the age of 20 years. By the end or during their third decade, almost all patients (99%) develop at least the pathognomonic mucocutaneous lesions.46, 47, 48, 49 Diagnostic criteria for CS have been established by the International Cowden's Consortium CD Diagnosis Criteria/National Comprehensive Cancer Network.
Affected individuals with CS will develop both benign and malignant tumors in a variety of tissues, such as breast, uterus, and thyroid. Thyroid pathologic findings in this syndrome typically affect follicular cells.50, 51 Follicular carcinoma, with a frequency of 5–10%, is a major diagnostic criterion for the diagnosis of CS; multinodular goiter (nodular hyperplasia), multiple adenomatous nodules, and follicular adenomas are minor criteria, with a frequency of 50–67%.
BRRS generally presents in childhood with delayed motor and intellectual development, other described features include thyroid adenomas, lymphocytic thyroiditis/Hashimoto's thyroiditis, lymphatic/vascular malformations, high arched palate, scoliosis, seizures, lipid storage myopathy, and joint hyperextensibility. There is no international criteria for the diagnosis of BRRS, but Marsh et al49 defined at least three of the following four features: macrocephaly, lipomatosis, hemangiomas, and speckled penis in males, whereas Parisi52 defined it as at least two of three features of macrocephaly, hamartomas (at least one lipoma, hemangioma, or intestinal polyp), and penile macules in males.
Multiple thyroid nodules, adenomatous nodules, are a characteristic finding in PHTS. Gross examination reveals multiple firm yellow-tan well-circumscribed nodules, diffusely involving the thyroid gland (Figure 8). Microscopically, they are well-circumscribed non-encapsulated solid cellular nodules sharing features similar to follicular adenomas. Follicles are not dilated, and some nodules may have a discontinuous rim of fibrous tissue simulating a capsule (Figure 9). The nodules range in size from 0.1 up to 8 cm and the number of nodules per thyroid can be over 100 distinct nodules.50, 51, 53
Follicular adenomas are very common and usually multiple in this syndrome. Follicular carcinoma is an important feature in CS and BRRS; these tumors are more frequently multicentric, and progress from a pre-existing follicular adenoma. The majority of thyroid lesions occurring in PHTS are follicular carcinoma; PTC is rarely been associated with this entity.50, 53 The occurrence of papillary microcarcinoma in CS is usually considered to be a chance occurrence as such tumors may be present in up to 30% of the adult population.50
MTC is not considered as a part of the spectrum of PHTS; however, previous studies, including studies conduct by us50, 53 and others,51 have identified CCH in individuals affected with this syndrome. The presence of CCH and an abnormal distribution of C cells are seen in PHTS. Two types of CCH that differ by their characteristics are identified: neoplastic CCH and reactive or physiological CCH. CCH associated with familial C-cell-related syndromes is usually both diffuse and nodular. Neoplastic CCH progresses to MTC; these tumors in its hereditary form are preceded by CCH. Reactive CCH is considered to be caused by a stimulus that is external to the C cell, and its premalignant potential is not documented. These are usually associated with diffuse CCH. Many situations such as hyperparathyroidism, hypercalcemia, lymphocytic thyroiditis, or follicular tumors have been associated with reactive CCH.9, 10 Although the significance of CCH in PHTS is unknown, these findings suggest that PTEN-associated tumor syndromes should be considered in the differential diagnosis of CCH of the thyroid.
We have recently reviewed50 20 thyroid pathology cases in 14 females and 6 males with PHTS (CS, 14 cases; BRRS, 6 cases). The majority of cases had family history of PHTS. The mean age of diagnosis of PHTS was 33.7 years, that of BRRS was 15 years, and 41.7 years for CS. The thyroid lesions were diagnosed around the same time, as these patients were diagnosed as PHTS.
Each thyroid had multiple findings including multiple adenomatous nodules, papillary carcinoma, follicular carcinoma, multiple follicular adenomas, marked lymphocytic thyroiditis, and CCH. The most common diagnosis was multiple adenomatous nodules (75%), followed by papillary carcinoma (60%), lymphocytic thyroiditis (55%), follicular carcinoma (45%), nodular hyperplasia (25%), solitary follicular adenomas (15%), and multiple follicular adenomas (10%). CCH was found interestingly in 67% of cases of BRRS and in 21% of CS.
Most studies have failed to show a consistent genotype–phenotype relationship in PHTS. Careful phenotyping gives further support for the suggestion that BRRS and CS are actually one condition, presenting at different stages.38 Our study has confirmed these observations.
In summary, the presence of numerous adenomatous nodules in a background of lymphocytic thyroiditis in younger patients should raise the suspicion for the diagnosis of PHTS. Multicentric thyroid pathology characterized by the presence of numerous adenomatous nodules, follicular adenomas, and the presence of FTC is a characteristic thyroid pathology finding of PHTS.
We suggest a diagnostic approach in an enlarged, bilateral, and multifocal multinodular thyroid disease in PHTS (Figure 10).
Carney's complex
Carney's complex is an autosomal dominant disease, characterized by skin and mucosal pigmentation, diverse pigmented skin lesions, non-endocrine, and a variety of endocrine neoplasias (pituitary adenoma, pigmented nodular adrenal disease, Sertoli and Leydig cell tumors, and thyroid tumors).
Patients with Carney's complex may share similar components with other familial MEN. The thyroid is usually multinodular with multiple adenomatous nodules, follicular adenomas, and both PTC and FTC are present in about 15% of patients with Carney's complex.54, 55
Werner's syndrome
Werner's syndrome is an autosomal recessive connective tissue disease, characterized by premature aging, bilateral cataracts, gray hair, and skin atrophy.
Patients with this syndrome have increased risk of a variety of neoplasias, including benign thyroid lesions and an increased incidence of PTC (only tumor present in white patients), and the most common tumor in Japanese patients (84%), followed by FTC (14%) and anaplastic thyroid carcinomas (2%). This latter neoplasm occurs in this syndrome at a higher frequency as compared with the general population.56, 57, 58
Multiple endocrine neoplasia IIA
The frequency of microscopic PTC is approximately twice as great in thyroid glands of MEN IIA patients if compared with general population. These cases usually present with multiple microscopic PTCs. These microcarcinomas are likely to carry only modest clinical significance, as microscopic PTCs often remain clinically silent and affected subjects carrying germline RET mutations undergo thyroidectomy at a young age.59, 60
Familial Tumor Syndromes Characterized by a Predominance of Non-medullary Thyroid Carcinoma
FNMTC syndrome is diagnosed when three or more family members have non-medullary thyroid cancer in the absence of other known associated syndromes. Statistical estimates suggest that a grouping of two family members with NMTC could represent the concurrence of sporadic tumors, but thyroid tumors in three or more members in kindred, or the diagnosis of PTC in men and children, is more suggestive of a familial predisposition.24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 61
Although non-medullary thyroid cancer is mostly sporadic, evidence for a familial form, not associated with other Mendelian cancer syndromes described above (eg FAP and CS), is well documented and thought to cause more aggressive disease. The search for a genetic susceptibility locus for FNMTC started about a decade ago.
FNMTC is now recognized as a distinct clinical entity and accounts for up to 10.5% of all follicular cell origin thyroid carcinomas. FNMTC has a high incidence of multifocality and association with multiple benign nodules. FNMTC patients have shorter disease-free survival than do sporadic disease patients because of frequent locoregional recurrence.24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 61
The genetic inheritance of FNMTC remains unknown, but it is believed to be an autosomal dominant mode with incomplete penetrance and variable expressivity. Genetic analyses of large FNMTC kindreds not only support the hypothesis of an inherited genetic predisposition to FNMTC, but also represent the first steps in identification of the putative susceptibility genes.62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74 Six potential regions for harboring an FNMTC gene have been identified: MNG1 (14q32), thyroid carcinoma with oxyphilia (TCO) (19p13.2), fPTC/papillary renal neoplasia (PRN) (1q21), NMTC1 (2q21), FTEN (8p23.1–p22), and the telomere–telomerase complex. Linkage analyses have identified three different chromosomal regions that may harbor an FNMTC susceptibility gene.
Familial papillary thyroid carcinoma
Familial PTC (fPTC) is characterized by multicentric tumors and multiple adenomatous nodules with or without oxyphilia (Figure 11). The fPTC enriched in TCO has been mapped to chromosomal region 19p13, and FNMTC without oxyphilia has also been mapped to 19p13.31. Tumor-specific loss of heterozygosity is found in sporadic FTC with and without oxyphilia at both 19p13 and 2q21.32.27, 63, 64
FNMTC type 1 syndrome
The FNMTC type 1 syndrome (chromosomal region 2q21) is characterized by PTC without any distinguishing pathologic features and without an obvious increase in frequency of non-thyroidal neoplasms in kindred members.70
fPTC associated with renal papillary neoplasia
The fPTC associated with renal papillary neoplasia presents with the usual classical variant of PTC and with no special features. The PRN syndrome (fPTC/PRN), mapped to chromosomal region 1q21, includes not only PTC and the expected benign thyroid nodules, but also papillary renal neoplasia and possibly other malignancies as well.71
Familial multinodular goiter syndrome
In familial multinodular goiter syndrome, which is mapped to 14q, some patients may develop an associated PTC.61, 72
FNMTC has been shown to be associated with the presence of multiple benign nodules, to behave in a more aggressive clinical behavior, and to have a worse prognosis than sporadic non-medullary thyroid cancer. Individuals with FNMTC have an increased risk of multifocal disease, local invasion, and increased local or regional recurrence and lymph node metastases. Compared with the patients with sporadic disease, the FNMTC patients were more likely to have intraglandular dissemination. FNMTC is an independent predictor of shorter disease-free survival.30, 31, 32
PTC in FNMTC has a well-documented predisposition to
-
multicentric disease
-
bilateral disease
-
local invasion
-
extrathyroidal extension
-
lymph node metastases
-
recurrence
-
specific histology.
The background thyroid may show
-
lymphocytic thyroiditis
-
multinodular hyperplasia
-
multiple adenomatous nodules.
However, despite common features, familial thyroid carcinomas are heterogeneous, show diverse natural histories, and require better characterization in distinguishing one type from another. FNMTCs are a relatively more aggressive disease. There a few publications related to FNMTC and primarily concerning prognosis and treatment. There are a few reports on the pathology of the tumors in the different syndromes.1, 2, 73
Sporadic PTC has BRAF mutation in approximately 40% of cases; however, no BRAF mutation was reported in a group of 40 patients with FNMTC as germline mutation or a susceptibility genetic event for FNMTC.74
Genetic studies on NMTC have been on small family groups using varying criteria for the diagnosis of FNMTC. The results have been contradictory and further large-scale genetic studies using emerging molecular screening tests are warranted to elucidate the underlying genetic basis of FNMTC. The genetics of FNMTC has the potential to permit individualized management of thyroid cancer.
A recent review on the genetics of familial non-medullary thyroid cancer75 performed through review of references from the English literature is available and confirmed six potential regions for harboring an FNMTC gene: MNG1 (14q32), TCO (19p13.2), fPTC/PRN (1q21), NMTC1 (2q21), FTEN (8p23.1–p22), and the telomere–telomerase complex. Important genes reported to have been excluded are RET, TRK, MET, APC, PTEN, and TSHR.
The systematic use of an algorithmic approach will help in the understanding of familial history and findings, and patient's clinical history associated with the pathology findings to suggest a familial disease (Figure 12). The clinical features and relative frequency of different forms of familial thyroid cancer have been discussed and presented recently.76
The clinical features associated with familial thyroid cancer syndrome are variable according to the syndrome the thyroid disease and cancer presented with (Table 7). The relative frequency of different forms of familial thyroid cancer has been discussed and presented recently by Vriens et al.76
Conclusions
Approximately 25% of MTCs are familial, and genotypic–phenotype correlation is well established. Hereditary MTCs usually occur as part of MEN II syndromes or as the MTC-only syndrome. MEN IIA is associated with pheochromocytoma and parathyroid hyperplasia, whereas MEN IIB is associated with marfanoid habitus, mucosal neuromas, ganglioneuromatosis, and pheochromocytoma. The hereditary tumor is usually preceded by CCH. Sporadic MTC usually is unilateral. MTC is in association with MEN syndromes, it is always bilateral and multicentric. All patients with MTC should then be screened for familial disease. Genetic testing is considered the standard of care for all first-degree relatives of patients with newly diagnosed MTC.
However, the familial inheritance of follicular-derived carcinomas is still unknown and represents 5–15% of the NMTCs, and constitutes a diversity of tumors and tumor syndromes. We define and separate follicular cell-derived familial thyroid carcinomas into two distinct groups of diseases. The first group is characterized by an increased prevalence of NMTC within a familial cancer syndrome or familial tumor syndromes characterized by a preponderance of non-thyroidal tumors. Within the second group, the predominant neoplasm is an NMTC, so-called familial tumor syndromes characterized by a predominance of NMTC. The entity of FNMTC is defined by the presence of follicular cell-derived thyroid tumors in three or more first-degree relatives. In general, the tumors in FNMTC are associated with early age of onset, usually associated with benign thyroid conditions, increased incidence of multifocality, bilateral tumors, nodal involvement, and recurrence, with some having a more aggressive behavior when compared with sporadic tumors. The gene for fPTC is yet to be identified, and no genetic test is presently available. The FNMTC as part of familial syndromes, characterized by a predominance of non-thyroidal tumors, has known genetic inheritance. Within this category of diseases is FAP and PHTS, within others. The thyroid pathology findings of these tumors are distinct.
The characteristic thyroid pathology findings in some of these syndromes should alert the pathologist to a possible familial cancer syndrome, as the correct histological interpretation may lead to further molecular genetic evaluation of the patient and family members. Most of the patients with familial disease are asymptomatic and are discovered through genetic screening in predisposing families. The identification of hereditary cases and early diagnosis makes preventive surgery and adequate treatment possible. Improved awareness and screening of FNMTC will permit earlier detection, a timely intervention, and improved outcomes for patients and their families. The pathologist is an important link in identifying these inherited tumors, and may have a crucial part in a patient's care. The careful histopathological interpretation of familial thyroid carcinomas leads to further investigation of family history with the addition of genetic and molecular tests of affected patient and family.
References
Nosé V . Familial non-medullary thyroid carcinoma: an update. Endocr Pathol 2008;19:226–240.
Dotto J, Nosé V . Familial thyroid carcinoma: a diagnostic algorithm. Adv Anat Pathol 2008;15:332–349.
Ries LAG, Melbert D, Krapcho M, et al. 2007 SEER Cancer Statistics Review, 1975–2004. National Cancer Institute: Bethesda, MD http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site.
Davies L, Welch HG . Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006;295:2164–2167.
Leenhardt L, Grosclaude P, Cherie-Challine L . Increased incidence of thyroidcarcinoma in France: a true epidemic or thyroidnodule management effects? Report from the French Thyroid Cancer Committee. Thyroid 2004;14:1056–1060.
Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53,856 cases of thyroidcarcinoma treated in the U.S., 1985–1995 [see comments]. Cancer 2004;83:2638–2648.
DeLellis RA, Lloyd RV, Heitz PU, et al. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. IARC Press: Lyon, 2004.
Mears L, Diaz-Cano SJ . Difference between familial and sporadic medullary thyroid carcinomas. Am J Surg Pathol 2003;27:266–267.
Wolfe HJ, DeLellis RA . Familial medullary thyroid carcinoma and C cell hyperplasia. Clin Endocrinol Metab 1981;10:351–365.
Perry A, Molberg K, Albores-Saavedra J . Physiologic versus neoplastic C-cell hyperplasia of the thyroid: separation of distinct histologic and biologic entities. Cancer 1996;77:750–756.
Chiefari E, Russo D, Giuffrida D, et al. Analysis of RET proto-oncogene abnormalities in patients with MEN 2A, MEN 2B, familial or sporadic medullary thyroid carcinoma. J Endocrinol Invest 1998;21:358–364.
Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 1996;276:1575–1579.
Eng C . Seminars in medicine of the Beth Israel Hospital, Boston. The RET proto-oncogene in multiple endocrine neoplasia type 2 and Hirschsprung's disease. N Engl J Med 1996;335:943–951.
Eng C, Mulligan LM, Healey CS, et al. Heterogeneous mutation of the RET proto-oncogene in subpopulations of medullary thyroid carcinoma. Cancer Res 1996;56:2167–2170.
Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994;367:375–376.
Komminoth P, Kunz EK, Matias-Guiu X, et al. Analysis of RET proto-oncogenepoint mutations distinguishes heritable from nonheritable medullary thyroid carcinomas. Cancer 1995;76:479–489.
Matias-Guiu X . RET protooncogene analysis in the diagnosis of medullary thyroid carcinoma and multiple endocrine neoplasia type II. Adv Anat Pathol 1998;5:196–201.
Dvorakova S, Vaclavikova E, Sykorova V, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinomas. Mol Cell Endocrinol 2008;284:21–27.
Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 2008;93:682–687.
Kebebew E, Ituarte PH, Siperstein AE, et al. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000;88:1139–1148.
Machens A, Niccoli-Sire P, Hoegel J, et al. Early malignant progression of hereditary medullary thyroid cancer. N Engl J Med 2003;349:1517–1525.
Romei C, Elisei R, Pinchera A, et al. Somatic mutations of the ret protooncogene in sporadic medullary thyroid carcinoma are not restricted to exon 16 and are associated with tumor recurrence. J Clin Endocrinol Metab 1996;81:1619–1622.
Alsanea O, Clark OH . Familial thyroid cancer. Curr Opin Oncol 2001;13:44–45.
Charkes ND . On the prevalence of familial nonmedullary thyroid cancer in multiple affected kindreds. Thyroid 2006;16:181–186.
Hemminki K, Eng C, Chen B . Familial risks for nonmedullary thyroid cancer. J Clin Endocrinol Metab 2005;90:5747–5753.
Leprat F, Bonichon F, Guyot M, et al. Familial non-medullary thyroid carcinoma: pathology review in 27 affected cases from 13 French families. Clin Endocrinol 1999;50:589–594.
Musholt TJ, Musholt PB, Petrich T, et al. Familial papillary thyroid carcinoma: genetics, criteria for diagnosis, clinical features, and surgical treatment. World J Surg 2000;24:1409–1417.
Ron E, Kleinerman RA, LiVolsi VA, et al. Familial nonmedullary thyroid cancer. Oncology 1991;48:309–311.
Sturgeon C, Clark OH . Familial nonmedullary thyroid cancer. Thyroid 2005;15:588–593.
Uchino S, Noguchi S, Kawamoto H, et al. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg 2002;26:897–902.
Alsanea O, Wada N, Ain K, et al. Is familial non-medullary thyroid carcinoma more aggressive than sporadic thyroid cancer? A multicenter series. Surgery 2000;128:1043–1050, discussion 1050–1051.
Grossman RF, Tu SH, Duh QY, et al. Familial nonmedullary thyroid cancer. An emerging entity that warrants aggressive treatment. Arch Surg 1995;130:892–897; discussion 898–899.
Lupoli G, Vitale G, Caraglia M, et al. Familial papillary thyroid microcarcinoma: a new clinical entity. Lancet 1999;353:637–639.
Malchoff CD, Malchoff DM . Familial nonmedullary thyroid carcinoma. Cancer Control 2006;13:106–110.
Malchoff CD, Sarfarazi M, Tendler B, et al. Familial papillary thyroid carcinoma is genetically distinct from familial adenomatous polyposis coli. Thyroid 1999;9:247–252.
Takami H, Ozaki O, Ito K . Familial nonmedullary thyroid cancer: an emerging entity that warrants aggressive treatment. Arch Surg 1996;131:676.
Triponez F, Wong M, Sturgeon C, et al. Does familial nonmedullary thyroid cancer adversely affect survival? World J Surg 2006;30:787–793.
Harach HR, Williams GT, Williams ED . Familial adenomatous polyposis associated thyroid carcinoma: a distinct type of follicular cell neoplasm. Histopathology 1994;25:549–561.
Soravia C, Sugg SL, Berk T, et al. Familial adenomatous polyposis-associated thyroid cancer: a clinical pathological, and molecular genetics study. Am J Pathol 1999;154:127–135.
Cameselle-Teijeiro J, Chan JK . Cribriform-morular variant of papillary carcinoma: a distinctive variant representing the sporadic counterpart of familial adenomatous polyposis-associated thyroid carcinoma? Mod Pathol 1999;12:400–411.
Herraiz M, Barbesino G, Faquin W, et al. Prevalence of thyroid cancer in familial adenomatous polyposis syndrome and the role of screening ultrasound examinations. Clin Gastroenterol Hepatol 2007;5:367–373.
Chung DC, Maher MM, Faquin WC . Case records of the Massachusetts General Hospital. Case 37-2006. A 19-year-old woman with thyroid cancer and lower gastrointestinal bleeding. N Engl J Med 2006;355:2349–2357.
Cetta F, Montalto G, Gori M, et al. Germline mutations of the APC gene in patients with familial adenomatous polyposis-associated thyroid carcinoma: results from a European cooperative study. J Clin Endocrinol Metab 2000;85:286–292.
Uchino S, Noguchi S, Yamashita H, et al. Mutational analysis of the APC gene in cribriform-morula variant of papillary thyroid carcinoma. World J Surg 2006;30:775–779.
Xu B, Yoshimoto K, Miyauchi A, et al. Cribriform-morula variant of papillary thyroid carcinoma: a pathological and molecular genetic study with evidence of frequent somatic mutations in exon 3 of the β-catenin gene. J Pathol 2003;199:58–67.
Zbuk KM, Eng C . Cancer phenomics: RET and PTEN as illustrative models. Nat Rev Cancer 2007;7:35–45.
Marsh DJ, Coulon V, Lunetta KL, et al. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet 1998;7:507–515.
Eng C . PTEN: one gene, many syndromes. Hum Mutat 2003;22:183–198.
Marsh DJ, Kum JB, Lunetta KL, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet 1999;8:1461–1472.
Laury A, Nosé V . Thyroid pathology in PTEN hamartoma tumor syndrome (PHTS): characteristic findings of a distinct entity (personal communication).
Harach HR, Soubeyran I, Brown A, et al. Thyroid pathologic findings in patients with Cowden disease. Ann Diagnost Pathol 1999;3:331–340.
Parisi MA, Dinulos MB, Leppig KA, et al. The spectrum and evolution of phenotypic findings in PTEN mutation positive cases of Bannayan-Riley-Ruvalcaba syndrome. J Med Genet 2001;38:52–58.
Zambrano E, Holm I, Glickman J, et al. Abnormal distribution and hyperplasia of thyroid C-cells in PTEN-associated diseases. Endoc Pathol 2004;15:55–64.
Stratakis CA, Courcoutsakis NA, Abati A, et al. Thyroid gland abnormalities in patients with the syndrome of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas (Carney complex). J Clin Endocr Metab 1997;82:2037–2043.
Stratakis CA, Kirschner LS, Taymans SE, et al. Carney complex, Peutz-Jeghers syndrome, Cowden disease, and Bannayan-Zonana syndrome share cutaneous and endocrine manifestations, but not genetic loci. J Clin Endocr Metab 1998;83:2972–2976.
Goto M, Miller RW, Ishikawa Y, et al. Excess of rare cancers in Werner syndrome (adult progeria). Cancer Epidemiol Biomark Prevent 1996;5:239–246.
Ishikawa Y, Sugano H, Matsumoto T, et al. Unusual features of thyroid carcinomas in Japanese patients with Werner syndrome and possible genotype-phenotype relations to cell type and race. Cancer 1999;85:1345–1352.
Nehlin JO, Skovgaard GL, Bohr VA . The Werner syndrome. A model for the study of human aging. Ann N Y Acad Sci 2000;908:167–179.
Giacomelli L, Guerriero G, Falvo L, et al. Simultaneous occurrence of medullary carcinoma and papillary microcarcinoma of thyroid in a patient with MEN 2A syndrome. report of a case. Tumori 2007;93:109–111.
Biscolla RP, Ugolini C, Sculli M, et al. Medullary and papillary tumors are frequently associated in the same thyroid gland without evidence of reciprocal influence in their biologic behavior. Thyroid 2004;14:946–952.
Bakhsh A, Kirov G, Gregory GW, et al. A new form of familial multi-nodular goitre with progression to differentiated thyroid cancer. Endocr Relat Cancer 2006;13:475–483.
Bevan S, Pal T, Greenberg CR, et al. A comprehensive analysis of MNG1, TCO1, fPTC, PTEN, TSHR, and TRKA in familial nonmedullary thyroid cancer: confirmation of linkage to TCO1. J Clin Endocrinol Metab 2001;86:3701–3704.
Burgess JR, Duffield A, Wilkinson SJ, et al. Two families with an autosomal dominant inheritance pattern for papillary carcinoma of the thyroid. J Clin Endocrinol Metab 1997;82:345–348.
Lesueur F, Stark M, Tocco T, et al. Genetic heterogeneity in familial nonmedullary thyroid carcinoma: exclusion of linkage to RET, MNG1, and TCO in 56 families. NMTC Consortium. J Clin Endocrinol Metab 1999;84:2157–2162.
Eng C . The role of PTEN, a phosphatase gene, in inherited and sporadic nonmedullary thyroid tumors. Recent Progr Hormone Res 1999;54:441–452; discussion, 453.
Canzian F, Amati P, Harach HR, et al. A gene predisposing to familial thyroid tumors with cell oxyphilia maps to chromosome 19p13.2. Am J Hum Genet 1998;63:1743–1748.
Leprat F, Bonichon F, Guyot M, et al. Familial thyroid carcinoma: pathology review in 27 affected cases from 13 French families. Clin Endocrinal 1999;50:589–594.
Katoh R, Harach HR, Williams ED . Solitary, multiple, and familial oxyphil tumours of the thyroid gland. J Pathol 1998;186:292–299.
McKay JD, Williamson J, Lesueur F, et al. At least three genes account for familial papillary thyroid carcinoma: TCO and MNG1 excluded as susceptibility loci from a large Tasmanian family. Eur J Endocrinol 1999;141:122–125.
McKay JD, Lesueur F, Jonard L, et al. Localization of a susceptibility gene for familial nonmedullary thyroid carcinoma to chromosome 2q21. Am J Hum Genet 2001;69:440–446.
Malchoff CD, Sarfarazi M, Tendler B, et al. Papillary thyroid carcinoma associated with papillary renal neoplasia: genetic linkage analysis of a distinct heritable tumor syndrome. J Clin Endocrinol Metab 2000;85:1758–1764.
Bignell GR, Canzian F, Shayeghi M, et al. Familial nontoxic multinodular thyroid goiter locus maps to chromosome 14q but does not account for familial nonmedullary thyroid cancer. Am J Hum Genet 1997;61:1123–1130.
Harach R . Familial nonmedullary thyroid neoplasia. Endocr Pathol 2001;12:97–112.
Xing M . The T1799A BRAF mutation is not a germline mutation in familial nonmedullary thyroid cancer. Clin Endocrinol (Oxf) 2005;63:263–266.
Khan A, Smellie J, Nutting C, et al. Familial nonmedullary thyroid cancer: a review of the genetics. Thyroid 2010;20:795–801.
Vriens MR, Suh I, Moses W, et al. Clinical features and genetic predisposition to hereditary nonmedullary thyroid cancer. Thyroid 2009;19:1343–1349.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Additional information
Partially presented at USCAP Long Course on Endocrine Pathology, Washington, DC, March 2010.
Rights and permissions
About this article
Cite this article
Nosé, V. Familial thyroid cancer: a review. Mod Pathol 24 (Suppl 2), S19–S33 (2011). https://doi.org/10.1038/modpathol.2010.147
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.147
Keywords
This article is cited by
-
Is unicentric familial papillary thyroid microcarcinoma different from multicentric?
Endocrine (2023)
-
Molecular profiling of papillary thyroid carcinomas in healthcare workers exposed to low dose radiation at the workplace
Endocrine (2022)
-
Biological behavior of familial papillary thyroid microcarcinoma: Spanish multicenter study
Langenbeck's Archives of Surgery (2022)