Abstract
The Turin Proposal algorithm defines poorly differentiated carcinoma on the basis of the presence of solid/trabecular/insular growth pattern, absence of conventional nuclear features of papillary carcinoma, and the presence of at least one of the following features: convoluted nuclei, mitotic activity ≥3/10 HPF, or tumor necrosis. IMP3 appears to have diagnostic and prognostic value in many solid tumors, including thyroid carcinomas. We examined a series of follicular-cell carcinomas with prominent solid patterns diagnosed at Mayo Clinic (56 cases) (Rochester, MN, USA) and at the University of Turin (96 cases) (Northern Italy) to validate the Turin consensus criteria defining poorly differentiated carcinoma of the thyroid and to evaluate the prevalence and prognostic behavior of this tumor. On this series, we analyzed the expression of conventional markers by immunohistochemistry and we investigated the expression of IMP3 by both immunohistochemistry and qRT-PCR. The prevalence of poorly differentiated carcinoma among the USA cases was 1.8% (56/3128) and that in the cases of Northern Italy was 6.7% (96/1442). Tumor characteristics were similar in the cases from the USA and from Italy except for extensive vascular invasion and a prevalent insular growth pattern (lower the former, higher the latter in the Italian series). In univariate analysis, the risk of death was higher for age ≥45, tumors ≥4 cm, and IMP3 positivity. Multivariate analysis showed that the risk of death from poorly differentiated carcinoma was higher for age ≥45. The Turin consensus criteria can reliably select poorly differentiated carcinomas. Tumors from the USA and from Italy showed similar overall survival, although the prevalence of poorly differentiated carcinoma was higher in Northern Italy. Expression of IMP3 appears to be an adverse prognostic factor for poorly differentiated thyroid carcinoma.
Similar content being viewed by others
Main
Poorly differentiated carcinoma of the thyroid is a worldwide recognized entity, but its incidence varies from reports of less than 1% of thyroid cancers in Japan1 and 2–3% of thyroid cancers in North America, to 15% in Northern Italy.2 These variations may either be real and related to geographic (environmental) influences or could be due to differences in histopathological interpretation.
The term ‘poorly differentiated carcinoma’ of the thyroid gland was proposed more than 25 years ago to define a follicular-cell carcinoma showing limited evidence of structural follicular or papillary features, and having a clinical behavior intermediate between follicular or papillary and anaplastic carcinomas. Different terms have been used to refer to these tumors, including solid,3 trabecular,4 insular,5, 6 poorly differentiated,3, 7, 8 intermediate-type,9 primordial cell,10 less well-differentiated carcinoma,11 or follicular carcinoma with insular component.12
In the latest World Health Organization (WHO) classification of Tumors of Endocrine Organs,13 poorly differentiated carcinoma was introduced as a separate entity and defined as a follicular-cell neoplasm showing limited evidence of structural follicular cell differentiation, and morphologically and behaviorally at an intermediate position between differentiated (follicular and papillary carcinomas) and undifferentiated (anaplastic) carcinoma.
An international consensus meeting was held in Turin, on March 2006, to develop uniform diagnostic criteria for poorly differentiated carcinomas. An international panel of 12 thyroid pathologists recommended a diagnostic algorithm (‘Turin Proposal criteria’) on the basis of the interpretation of the WHO classification and suggesting a uniform approach to classify these tumors. The Turin proposal algorithm defines poorly differentiated carcinomas by the presence of solid/trabecular/insular growth pattern, absence of conventional nuclear features of papillary carcinoma, and the presence of at least one of the following features: convoluted nuclei, mitotic activity ≥3/10 HPF, or tumor necrosis.14
Recently, Ito et al15 in Japan compared the prognostic value of different histopathological definitions in a large series of thyroid malignancies and they concluded that in their series the subgroup defined according to the Turin consensus criteria was an uncommon but well-defined entity and included a subset of thyroid carcinomas associated with a more aggressive behavior. Volante et al16 analyzed the prognostic impact of several clinicopathological parameters in a large series of poorly differentiated carcinomas classified according to the pattern of growth (trabecular, insular, and/or solid) and reported that age ≥45 years, presence of necrosis, and mitotic count ≥3 × 10 HPF were associated with poor outcome. In agreement with these findings, the study by Hiltzik et al,17 reported that poorly differentiated carcinomas defined on the basis of high mitotic activity and/or tumor necrosis have a prognosis intermediate between well-differentiated thyroid carcinomas and anaplastic tumors, and also found that the growth pattern was not predictive of survival in this group of patients. More recently, poorly differentiated carcinomas with necrosis and/or mitotic index ≥5 × 10 HPF, irrespective of the growth pattern, were identified as radioactive iodine-refractory tumors.18
With this background, we retrieved a series of follicular-cell carcinomas of the thyroid with prominent solid pattern from Mayo Clinic (Rochester, MN, USA) and from University of Turin (Turin, Italy) to validate the histological features of poorly differentiated carcinoma using the Turin proposal criteria, to better define the prevalence and evolution of this entity, and to analyze the prognostic effect of previously reported morphological or genetic parameters. In addition, we investigated the expression and potential prognostic role in poorly differentiated carcinoma of insulin-like growth factor-II mRNA binding protein-3 (IMP3), also known as L523S or KOC (K-homologous domain-containing protein over expressed in cancer), which is a member of the IMP family19 and has been reported as an important biomarker for several malignancies.20 IMP3 expression has diagnostic and/or prognostic value in many malignant solid tumors.20, 21 In thyroid tumors, IMP3 expression has been proposed as a diagnostically useful parameter in differentiating malignant and benign follicular pattern lesions.21, 22 We sought to investigate whether IMP3 might have prognostic implications in poorly differentiated carcinomas.
Materials and methods
Patient Selection
Four thousand five hundred seventy primary thyroid malignancies from patients who underwent primary surgical treatment at Mayo Clinic (Rochester, MN, USA; 3128 cases) between 1955 and 2000, and at the Molinette Hospital & University of Turin (Northern Italy; 1442 cases), during the period 1974–2008 were reviewed. Specifically, one of the authors (S Asioli) spent 6 months (from 1 March 2009 to 31 August 2009) at Mayo Clinic (Rochester, MN, USA) as visiting clinician, funded by World Wide Style Projects, Fondazione CRT, University of Turin, Italy, and fulfilling the project she personally reviewed the slides of 3128 primary thyroid malignancies, extracting 148 cases of interest with a solid pattern, which were further reviewed with two authors (RV Lloyd, LA Erickson). The Italian cases (no. 1442) were all reviewed by two of the authors (M Volante and S Asioli) and the selected cases of interest were further checked by four authors (S Asioli, G Bussolati, RV Lloyd and LA Erickson). A total of 152 cases of poorly differentiated carcinoma were identified according to Turin consensus criteria from Mayo Clinic (Rochester, MN, USA; 56 cases) and the University of Turin (Italy; 96 cases).
The following selection criteria were used: correlation to the Turin consensus criteria,14 including presence of well-differentiated component in less than 25% of the tumor (follicular or papillary type) and availability of clinicopathological and follow-up data, and of pathological material (slides and paraffin blocks). Some of these cases had been included in previous studies.10, 14, 16, 23
Institutional Review Board Permission was obtained for the study.
The following features were analyzed: prevalence, age, sex, tumor size, necrosis, convoluted nuclei, mitoses, atypical mitoses, vascular invasion (either minimal: ≤3 foci of vascular invasion or extensive: 4 or more foci of vascular invasion), oncocytic features, pattern of growth.
Disease persistence, recurrence, and metastases were evaluated by chart review, imaging studies, or histopathological examination of resected recurrent tumors and/or metastases.
Immunohistochemistry
Immunohistochemical analysis for IMP3 (L523; Dako, Carpinteria, CA, USA) thyroglobulin (2H11/6E1 cocktail; Zymed, San Francisco, CA, USA), TTF1 (8G7G3/1; Dako), HBME-1 (HBME-1; Dako), p53 (DO7; Dako), and Ki67 (MIB1; Dako) was performed on poorly differentiated carcinoma cases according to the standard automated immunohistochemical procedure (Ventana XT autostainer; Ventana Medical Systems, Tucson, AZ, USA).
Interpretation of Immunoistochemical Stains
The immunohistochemical expression of IMP3 and the expression of p53 and HBME-1 were scored by considering both the intensity and the extent of the stain using a semi-quantitative scoring system. The criteria for intensity score were as follows: 0 for no staining; 1+ for weak staining; 2+ for moderate staining; and 3+ for strong staining. The criteria for scoring the extent of the stain were as follows: 0 for no staining; 1+ for staining <25% of neoplastic cells; 2+ for staining 25–50% of neoplastic cells; and 3+ for staining >50% of the neoplastic cells. The two scores were cumulated and a stain was considered positive with a combined immunohistochemical score higher than 2, whereas a final score of 2 or lower was considered negative.
Molecular Analysis
Molecular analysis for IMP3 and RAS mutations was focused on a subgroup of poorly differentiated carcinoma cases with a pure insular growth pattern. In particular, molecular analysis of IMP3 expression could be performed in 47 of these cases. All tumor samples were analyzed for IMP3 expression using quantitative RT-PCR (qRT-PCR) as described previously.22 IMP3 mRNA expression was normalized with phosphoglycerokinase (PGK) mRNA expression and the IMP3 expression levels were expressed as relative fold change (fold) compared to normal thyroid RNA and TPC1 (calibrator).
Molecular analysis of RAS mutational status was carried out in 32 cases: 14 of these cases had already been analyzed in the recent study on RAS significance in poorly differentiated carcinomas;23 to these, we added 18 new cases showing pure insular growth pattern. All tumor samples were analyzed for NRAS codon-61, HRAS codon-61, and KRAS codon-12 and 13 point mutations using PCR amplification and DNA sequencing as described previously.23
Statistical Analysis
Continuous data were compared between groups using Wilcoxon rank-sum tests and categorical data were compared between groups using χ2 or Fisher's exact tests. The age of 45 years24 was considered as the cut-off age for statistical analyses. Survival time was defined as time from the date of first surgery to the time of death (or the last follow-up). We also looked at four other events: disease-free survival, time to recurrence, time to lymph node metastasis, and time to distant metastasis. The time to events were each compared between groups initially using log-rank tests and illustrated using Kaplan–Meier curves. Survival was also compared between groups using Cox proportional-hazards regression from which hazard ratios were estimated. In models where the number of events was very small in the level of one of the predictors, confidence intervals were calculated using the profile likelihood method, and likelihood ratio tests were used to calculate P-values. In all other cases, the Wald method was used for confidence intervals and P-values. Multivariable models were determined using stepwise variable selection. Associations between immunoreactivity with IMP3 and clinicopathological parameters were evaluated using the Yates corrected χ2 test for 2 × 2 tables. A comparison between immunohistochemistry and qRT-PCR IMP3 results was performed using Student's t-test. A Spearman correlation was used to look for a relationship between IMP3 molecular analysis results and the follow-up data. P-values less than 0.05 were considered statistically significant. All analyses were performed using SAS version 9 (Cary, NC, USA) and figures were generated in Splus (Palo Alto, CA, USA).
Results
The prevalence of poorly differentiated carcinomas in the Mayo Clinic series was 1.8% (56/3128) whereas in the University of Turin (Italy) series (a single Institution in Northern Italy) it was 6.7% (96/1442).
A summary of the associations between the clinicopathological parameters of the 152 cases between the two sites is provided in Table 1.
Clinical Features
The mean patient age was 60.6 years (median 61.4; range: 14.1–89.7). A female predominance was observed (female:male ratio, 1.6:1). Radioiodine therapy was administered (1–8 doses of 131I between 3700 and 7400 MBq) after total thyroidectomy in 74 patients for whom post-surgical treatment information was available.
Clinically aggressive tumors, including those with recurrences or metastatic spread and/or patient death, accounted for 99 of the 152 tumors (65%). Local recurrence was observed in 19 cases, metastases to lymph nodes in 22 cases, and to metastases to distant organs in 58 cases.
The estimated 5-year survival rate was 71.6% (95% CI: 64.0–80.1%) and the 10-year survival rate was 46.3% (95% CI: 37.4–57.3%) (Table 1).
Pathological Features
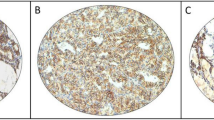
The mean tumor size was 5.9 cm (range: 2–13 cm). Most patients had vascular invasion, which was either minimal (≤3 foci of vascular invasion) in 50/152 (33%) or extensive (4 or more foci of vascular invasion) in 101/152 (66%) cases. Vascular invasion was identified in all but one patient who had, however, extensive full-thickness capsular penetration by the tumor. The type of growth pattern of the tumors included a predominantly insular pattern found in 58% (88/152) of the tumors and a solid-trabecular architecture in 42% (64/152) of the tumors. The presence of a well-differentiated component (≤25% of the tumor) as follicular carcinoma was found in 36 tumors (24%), whereas papillary thyroid carcinoma was identified in 16 tumors (11%). Predominant oncocytic features were identified in 48/152 tumors (32%). Convoluted nuclei were observed in 49/152 (32%) cases. Necrotic areas were present in 107/152 tumors (70%) (Figure 1a). A mitotic count ≥3 per 10 HPF was observed in 143/152 (94%) and atypical mitoses were present in 30/152 (20%) cases (Figure 1b).
An example of a poorly differentiated carcinoma case on the H&E showing a prominent insular growth pattern, necrosis (a, × 10), and high mitotic count (b, × 20). The peculiar pattern of dot-like paranuclear staining for thyroglobulin is observed in the majority of poorly differentiated carcinoma cells (c, × 20). A prevalent cytoplasmic staining for IMP3 is present in the poorly differentiated carcinoma cells whereas the adjacent normal thyroid cells are negative (d, × 10).
On the basis of these distinct morphological features and considering that all cases were meeting the Turin consensus criteria, we divided the poorly differentiated carcinoma cases into four groups.
Group-1 included 52/152 (34%) poorly differentiated carcinoma cases that showed a pure insular growth pattern and absence of even focal conventional nuclear features of papillary carcinoma. Group-2 included 16/152 (10%) poorly differentiated carcinoma cases that showed foci (<25% of the tumor) of well-differentiated papillary thyroid carcinoma. Group-3 included 36/152 (24%) poorly differentiated carcinoma cases that showed foci of well-differentiated follicular carcinoma in less than 25% of the tumors. Group-4 was composed of 48/152 (32%) poorly differentiated carcinoma cases that showed a solid/trabecular/insular growth pattern with oncocytic features in more than 75% of the tumors.
Tumor characteristics were similar between the Mayo Clinic and the University of Turin poorly differentiated carcinoma cases with the exception of extensive vascular invasion (84% at Mayo Clinic versus 57% at University of Turin, P=0.0009) and insular pattern (41% at Mayo Clinic versus 68% at University of Turin, P=0.001) (Table 1).
Immunohistochemical Analysis
Immunohistochemical analysis was feasible in 103 cases (68%) because of the limited quality (very old tissue blocks) of the tissue in 49 cases. Thyroglobulin was positive in 95 of 103 (92%) cases and showed a peculiar pattern of dot-like paranuclear staining in most cases (73/95) (77%) (Figure 1c). TTF1 immunostaining showed diffuse nuclear positivity in all 103 poorly differentiated carcinoma cases. HBME-1 showed focal membrane positivity in 50 of 103 cases (49%). P53 immunostaining showed nuclear positivity focally in 39 of 103 cases (38%) and diffuse nuclear positivity in 46 of 103 cases (45%). The Ki67 proliferative index ranged from 3 to 40%, with a mean of 13% (9.5 s.d.).
Sixty-one of 103 (59%) poorly differentiated carcinoma cases showed prevalent cytoplasmic staining for IMP3 (Figures 1d, 2d). Forty-two of 103 (41%) cases were considered negative because tumor cells were completely negative for IMP3 or showed immunohistochemical cumulative score less than 2. Tumor characteristics were compared between poorly differentiated carcinoma cases that were positive (n=61) versus those that were negative (n=42) for IMP3. No association could be established between IMP3 expression and sex, age, tumor size, angioinvasion, oncocytic features, presence of convoluted nuclei, pattern of growth, necrosis, and number of mitoses or atypical mitoses.
Molecular Analysis
IMP3 mRNA quantitative analysis
IMP3 mRNA quantitative analysis was only performed for poorly differentiated carcinoma cases of group-1 to avoid the risk of contamination by RNA belonging to well-differentiated tumor areas (papillary in group-2 and follicular in group-3) or oncocytic tumor cells (group-4). IMP3 was detected in 42 of 47 poorly differentiated carcinoma cases tested, with the remaining five cases having insufficient RNA. The relative IMP3 expression in 42 cases of poorly differentiated carcinoma was on the average 17.5 (14.6 s.d.)-fold higher than the normal thyroid RNA (calibrator). The results of Spearman correlation suggest that the relationship between IMP3 molecular analysis results and follow-up data is statistically significant (ρ=0.4683, P=0.0018).
RAS mutation analysis
RAS mutations were identified in 6 of 32 cases (19%), including 14 cases reported previously.23 All but one mutation were in the NRAS gene, codon-61, with the Gln-to-Arg (Q61R, CAA_CGA) substitution. The remaining tumor had the NRAS Q61K mutation (Q61K, CAA_AAA).
No mutation was detected in the KRAS and HRAS genes.
Three poorly differentiated carcinoma cases showing NRAS Q61R and the case with NRAS Q61K mutations were positive for IMP3 by immunohistochemistry and had an average of 20.8 (15.8 s.d.) IMP3 fold changes in qRT-PCR whereas the remaining two mutated cases were negative for IMP3 at immunohistochemistry and had an average of 8.6 IMP3 fold changes (6.8- and 10.4-fold changes, respectively).
Survival analysis
Univariate analysis showed that the risk of death was higher for patients with poorly differentiated carcinoma whose age was ≥45 years (HR=4.26, P<0.01) and for those with tumors ≥4 cm (HR=1.93, P=0.04) (Figures 2a, b). There was no significant difference in the risk of death between poorly differentiated carcinoma cases from Mayo Clinic versus those from University of Turin. The presence of atypical mitoses was associated with higher risk of recurrence (HR=2.74, P=0.03) and risk of lymph node metastasis (HR=2.69, P=0.03). Age ≥45 was associated with higher risk of distant metastasis (HR=2.99, P=0.02). No significant differences in the risk of recurrence or metastases were found between the Mayo Clinic and University of Turin poorly differentiated carcinoma cases by univariate analysis. No significant differences in overall survival, disease-free survival, and metastasis (lymph nodes and distant organs)-free survival were found between the four groups by univariate analysis (Table 2).
IMP3 and Ki67 stains were analyzed statistically among the 103 poorly differentiated carcinoma cases.
IMP3 positivity was associated with an increased risk of death (HR for positive versus negative =6.97, P<0.01) (Figure 2c), metastases (HR for lymph node metastasis=7.09, P=0.02; HR for distant metastases=15.65, P<0.01), and with a decrease of disease-free survival.
MIB-1/Ki67 was marginally associated with an increased risk of recurrence (HR for 5 unit increase=1.59, P=0.048) and of lymph node metastases (HR=2.06, P=0.04).
The results of multivariate analysis are summarized in Table 3.
By multivariate analysis, age ≥45 (HR=4.81, P=0.001) was associated with death of disease whereas presence of convoluted nuclei (HR=0.52, protective, P=0.02) was associated with a better overall survival. Furthermore, the risk of distant metastasis was higher for patients with age ≥45 (HR=3.85, P=0.005) and was lower for cases with convoluted nuclei (HR=0.39, P=0.005).
Among the 103 poorly differentiated carcinoma cases in which IMP3 immunohistochemical analysis was available, IMP3 positivity was associated with higher risk of distant metastases (HR positive versus negative for distant metastases=36.7, P<0.001). IMP3 positivity was also associated with decreased overall survival (HR for IMP3 positive versus negative=8.96, P<0.001) and decreased disease-free survival (HR for IMP3 positive versus negative=56.08, P<0.001).
Multivariate Cox regression analysis found IMP3 expression to be a strong independent prognostic factor followed by presence of necrosis (Table 3).
qRT-PCR analyses were performed for 42 cases of poorly differentiated carcinoma for which IMP3 immunohistochemical studies had also been performed. The average number IMP3 mRNA transcripts detected in 14 cases of poorly differentiated carcinoma negative for IMP3 by immunohistochemistry was 9.4 (s.d. 6.6)-fold, whereas the average number of IMP3 mRNA transcripts in 28 cases of poorly differentiated carcinoma showing IMP3 positivity by immunohistochemistry was 21.4 (s.d. 14.3)-fold (P=0.02, Student's t-test).
Discussion
This study shows that the Turin consensus criteria can reliably diagnose poorly differentiated carcinomas in a large series of primary thyroid malignancies from different countries, and this definition has clinical, prognostic, and possible pathogenetic significance. We have validated on a large retrospective case series from the USA and Italy the algorithmic approach proposed by the Turin Consensus Meeting and have verified that the application of these criteria to daily practice is a sound approach for diagnosing poorly differentiated carcinomas. The prevalence of poorly differentiated carcinomas was higher in the mountainous areas of Northern Italy than in the USA (6.7 versus 1.8%, respectively), the prevalence in USA being close to the <1% prevalence reported in Japan.1 These findings indicate geographic differences in the prevalence of this tumor type, in agreement with a higher incidence in areas having iodine deficiency.
Poorly differentiated carcinomas are clinically intermediate between well-differentiated follicular or papillary carcinomas and anaplastic carcinomas: poorly differentiated carcinoma-affected patients had a mean 5-year survival of 71.6% and a 10-year survival of 46.3%. Furthermore, our results indicate that the inclusion of these tumors into a distinct category, comprehensive also of cases with oncocytic features, appears justified. It is noteworthy that the oncocytic variant of poorly differentiated carcinoma was not originally considered in the Turin Consensus Meeting despite the fact that thyroid carcinomas having prevalent oncocytic features and meeting the diagnostic criteria for poorly differentiated carcinomas had been reported in the literature.25, 26
In our study, no significant differences in overall survival, disease-free survival, and metastasis-free survival were found between the poorly differentiated carcinomas with oncocytic features and the other groups by univariate analysis. However, detailed studies on this variant are still lacking and would be useful to better clarify the histogenesis of such tumors.
Clearly, the Turin proposal is only focused on solid/trabecular/insular tumors and does not refer to non-solid tumors showing tumor necrosis/high mitotic activity. One such examples is represented by aggressive variants of papillary thyroid carcinoma.27
The diagnosis of poorly differentiated carcinoma is primarily made on morphological criteria in H&E-stained sections. Immunohistochemistry is usually not necessary to classify a lesion in this group, but can be helpful in the confirmation of follicular-cell derivation. In fact, the solid/trabecular/insular growth pattern in poorly differentiated carcinomas might be a source of difficulties in differential diagnosis from other malignant lesions within the thyroid, including medullary thyroid carcinoma, parathyroid neoplasms, and metastatic tumors. In this respect, when a suspicion is raised by the morphological appearance, immunohistochemistry is advisable to avoid possible pitfalls. In particular, a peculiar pattern of thyroglobulin staining as dot-like paranuclear deposits (as originally described by some of us28) was observed in most of the poorly differentiated carcinoma cases of the present series (77%).
In our series, immunohistochemical markers thought by some to be diagnostic of malignancies, such as HBME-1 and p53, were often positive in poorly differentiated carcinomas, although the distribution of the former marker was more focal than in well-differentiated thyroid carcinomas. Thus, the usefulness of these markers in poorly differentiated carcinoma diagnosis is very limited. Similarly, an increase of proliferation index is associated with decrease of differentiation in thyroid tumors,29 and Martyniak et al30 suggested that p53 and Ki67 stains can assist in cases where diagnosis of poorly differentiated carcinoma is suspected on the basis of growth pattern, but lacking convoluted nuclei, increased mitotic count, or necrosis. In our experience, Ki67 might thus replace mitotic count as a more objective means of evaluating cell proliferation, but in well-preserved tissues, only.
In our study, IMP3 expression can significantly predict overall survival (HR for IMP3 positive versus negative=8.96, P<0.001), disease-free survival (HR for IMP3 positive versus negative=56.08, P<0.001), and metastasis-free survival (HR positive versus negative=36.7, P<0.001) in poorly differentiated carcinoma of the thyroid. The identification of the IMP3 expression as a prognostic parameter segregating aggressive from more indolent poorly differentiated carcinoma cases may bear important clinical implications. IMP3 can be evaluated by immunohistochemistry on cytological specimens and could potentially be incorporated into the routine practice.
Recently, Volante et al23 showed that RAS mutations are present in a fraction of poorly differentiated carcinomas and may be clinically relevant for the prognosis of patients affected by poorly differentiated carcinoma. Our study, comprehensive of 32 cases (14 already reported in a previous study23), supports and extends the above finding: in fact NRAS gene mutations in codon-61 were found in 19% of the current poorly differentiated carcinoma cases. In addition, it is interesting that 4 of 6 poorly differentiated carcinoma cases showing NRAS gene mutations were immunoreactive for IMP3, with a high IMP3 mRNA fold change at qRT-PCR.
In conclusion, poorly differentiated carcinomas of the thyroid are clinically and histologically intermediate between well-differentiated follicular or papillary carcinomas and anaplastic carcinomas. This study of a large tumor series selected on the basis of the Turin consensus criteria shows that poorly differentiated carcinoma is present in different geographical areas and has a biological behavior that is distinct from well-differentiated and anaplastic carcinomas of the thyroid. Poorly differentiated carcinomas in the USA and Italy showed similar overall survival, although the prevalence of poorly differentiated carcinoma is higher in the mountainous areas of Northern Italy. In addition, the variant of poorly differentiated carcinoma with oncocytic features shares the same behavior as other poorly differentiated carcinomas. Therefore, the classification of poorly differentiated carcinoma into a distinct entity, which also encompasses oncocytic poorly differentiated carcinomas, appears justified.
Furthermore, identification of the prognostic parameters differentiating highly aggressive from rather indolent cases has important clinical implications. Interestingly, our study identified a potential new prognostic marker: in fact IMP3 was a predictor at poor prognosis of poorly differentiated carcinoma, in both univariate and multivariate analysis, and detection of IMP3 by immunohistochemistry appears to be a useful prognostic factor for poorly differentiated carcinoma and it has potential for application on cytological specimens.
References
Kakudo K, Bai Y, Katayama S, et al. Classification of follicular cell tumors of the thyroid gland: analysis involving Japanese patients from one institute. Pathol Int 2009;59:359–367.
Sanders Jr EM, LiVolsi VA, Brierley J, et al. An evidence-based review of poorly differentiated thyroid cancer. World J Surg 2007;31:934–945.
Sakamoto A, Kasai N, Sugano H . Poorly differentiated carcinoma of the thyroid. A clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer 1983;52:1849–1855.
Cabanne F, Gérard-Marchant R, Heimann R, et al. Malignant tumors of the thyroid gland. Problems of histopathologic diagnosis. Apropos of 692 lesions collected by the thyroid cancer Cooperative Group of the O.E.R.T.C. Ann Anat Pathol 1974;19:129–148.
Carcangiu ML, Zampi G, Rosai J . Poorly differentiated (‘insular’) thyroid carcinoma. A reinterpretation of Langhans’ ‘wuchernde Struma’. Am J Surg Pathol 1984;8:655–668.
Pilotti S, Collini P, Mariani L, et al. Insular carcinoma: a distinct de novo entity among follicular carcinomas of the thyroid gland. Am J Surg Pathol 1997;21:1466–1473.
Sobrinho-Simões M, Sambade C, Fonseca E, et al. Poorly differentiated carcinomas of the thyroid gland: a review of the clinicopathologic features of a series of 28 cases of a heterogeneous, clinically aggressive group of thyroid tumors. Int J Surg Pathol 2002;10:123–131.
Nishida T, Katayama S, Tsujimoto M, et al. Clinicopathological significance of poorly differentiated thyroid carcinoma. Am J Surg Pathol 1999;23:205–211.
Ljungberg O, Bondeson L, Bondeson AG . Differentiated thyroid carcinoma, intermediate type: a new tumor entity with features of follicular and parafollicular cell carcinoma. Hum Pathol 1984;15:218–228.
Papotti M, Botto Micca F, Favero A, et al. Poorly differentiated thyroid carcinomas with primordial cell component. A group of aggressive lesions sharing insular, trabecular, and solid patterns. Am J Surg Pathol 1993;17:291–301.
Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer 1979;15:1033–1041.
Ashfaq R, Vuitch F, Delgado R, et al. Papillary and follicular thyroid carcinomas with an insular component. Cancer 1994;73:416–423.
Sobrinho-Simões M, Albores-Saavedra J, Tallini G, et al. Poorly differentiated carcinoma. In: DeLellis RA, Lloyd RV, Heitz U, Eng C (eds). Pathology and Genetics. Tumours of Endocrine Organs. World Health Organization IARC Press: Lyon, 2004. pp 73–76.
Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol 2007;31:1256–1264.
Ito Y, Hirokawa M, Fukushima M, et al. Prevalence and prognostic significance of poor differentiation and tall cell variant in papillary carcinoma in Japan. World J Surg 2008;32:1535–1543.
Volante M, Landolfi S, Chiusa L, et al. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer 2004;100:950–957.
Hiltzik D, Carlson DL, Tuttle RM, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer 2006;106:1286–1295.
Rivera M, Ghossein RA, Schoder H, et al. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer 2008;113:48–56.
Nielsen J, Christiansen J, Lykke-Andersen J, et al. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol 1999;19:1262–1270.
Kapoor S . IMP3: a new and important biomarker of systemic malignancies. Clin Cancer Res 2008;14:5640.
Slosar M, Vohra P, Prasad M, et al. Insulin-like growth factor mRNA binding protein 3 (IMP3) is differentially expressed in benign and malignant follicular patterned thyroid tumors. Endocr Pathol 2009;20:149–157.
Jin L, Seys AR, Zhang S, et al. Diagnostic utility of IMP3 expression in thyroid neoplasms. A quantitative RT-PCR study. Diag Mol Pathol 2009;19:63–69.
Volante M, Rapa I, Gandhi M, et al. RAS mutations are the predominant molecular alteration in poorly differentiated thyroid carcinomas and bear prognostic impact. J Clin Endocrinol Metab 2009;94:4735–4741.
Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th edn. Springer: Chicago, 2010.
Papotti M, Torchio B, Grassi L, et al. Poorly differentiated oxyphilic (Hurthle cell) carcinomas of the thyroid. Am J Surg Pathol 1996;20:686–694.
Seoane JM, Cameselle-Teijeiro J, Romero MA . Poorly differentiated oxyphilic (Hürthle cell) carcinoma arising in lingual thyroid: a case report and review of the literature. Endocr Pathol 2002;13:353–360.
Asioli S, Erickson LA, Sebo TJ, et al. Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. Am J Surg Pathol 2010;34:44–52.
Pietribiasi F, Sapino A, Papotti M, et al. Cytologic features of poorly differentiated ‘insular’ carcinoma of the thyroid, as revealed by fine-needle aspiration biopsy. Am J Clin Pathol 1990;94:687–692.
Tallini G, Garcia-Rostan G, Herrero A, et al. Downregulation of p27KIP1 and Ki67/Mib1 labeling index support the classification of thyroid carcinoma into prognostically relevant categories. Am J Surg Pathol 1999;23:678–685.
Martyniak A, Nose V . Does p53 and MIB-1 immunostaining help in the diagnosis of poorly differentiated thyroid carcinoma using the Turin criteria [abstract]. Mod Pathol 2009;22:118A.
Acknowledgements
Sources of support: This work was supported by grants from the Italian Ministry of University(ex-60%), Ricerca Sanitaria Finalizzata Piedmont Region, World Wide Style Projects, Fondazione CRT, University of Turin (to SA). A Righi is part of and funded by the PhD programme ‘Scienze Biomediche ed Oncologia Umana: Tecniche avanzate di localizzazione dei tumori umani’, University of Turin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Asioli, S., Erickson, L., Righi, A. et al. Poorly differentiated carcinoma of the thyroid: validation of the Turin proposal and analysis of IMP3 expression. Mod Pathol 23, 1269–1278 (2010). https://doi.org/10.1038/modpathol.2010.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.117
Keywords
This article is cited by
-
DICER1 tumor predisposition syndrome: an evolving story initiated with the pleuropulmonary blastoma
Modern Pathology (2022)
-
Newly proposed survival staging system for poorly differentiated thyroid cancer: a SEER-based study
Journal of Endocrinological Investigation (2022)
-
Aggressive variants of follicular cell-derived thyroid carcinoma: an overview
Endocrine (2022)
-
The biological function of IGF2BPs and their role in tumorigenesis
Investigational New Drugs (2021)
-
SEOM clinical guideline thyroid cancer (2019)
Clinical and Translational Oncology (2020)