Abstract
Neuroendocrine differentiation in prostate cancer correlates with overall prognosis and disease progression after androgen-deprivation therapy, although its specific mechanisms are currently poorly understood. A role of Notch pathway has been reported in determining neuroendocrine phenotype of normal and neoplastic tissues. The aim of this study was to analyze whether this pathway might affect neuroendocrine differentiation in prostate cancer. Human achaete-scute homolog 1 (hASH1), a pivotal member of the Notch pathway, was investigated in 80 prostate cancers selected and grouped according to chromogranin A immunohistochemistry, as follows: prostate cancers without neuroendocrine differentiation, untreated (25 cases); prostate cancers with neuroendocrine differentiation, untreated (40 cases); prostate cancers with previous androgen-deprivation therapy, all having neuroendocrine differentiation (15 cases). Human ASH1 protein was analyzed by immunohistochemistry, whereas the presence of hASH1 mRNA transcripts was investigated on paraffin material by real-time PCR. By immunohistochemistry, hASH1 was colocalized with chromogranin A in neuroendocrine cells of normal prostatic gland. It was absent in all but one prostate cancers without neuroendocrine differentiation, whereas it was positive in 25% of prostate cancers with neuroendocrine differentiation/untreated, with a significant correlation with the extent of neuroendocrine features (P=0.02). Moreover, comparing untreated and treated prostate cancers with neuroendocrine differentiation, a positive association with androgen-deprivation therapy was observed (P=0.01). In prostate cancers with neuroendocrine differentiation, RNA analysis confirmed the association of higher transcript levels in androgen deprivation-treated compared with untreated patients (P=0.01). In addition, hASH1 mRNA analysis in microdissected chromogranin A-positive and chromogranin A-negative areas within the same tumor demonstrated a two- to sevenfold increase of hASH1 mRNA expression in chromogranin A-positive tumor cell populations.
Similar content being viewed by others
Main
Prostate cancer is the leading malignancy in the aging male population. Prostate cancer biology is notoriously heterogeneous, some tumors showing a very aggressive course leading to patient's death in few years, others depicting an indolent behavior and remaining silent during the whole life.1 Accurate prediction of prognosis is important to tailor the optimal therapeutic approach for individual patients. To date, tumor stage, grade and serum PSA (prostate-specific antigen) levels are the only validated prognostic parameters.
Several prostate cancers display focal neuroendocrine differentiation at diagnosis, usually revealed by immunohistochemistry as scattered individual cells or nests of cells in the context of otherwise conventional adenocarcinomas.2 The biological significance of this phenomenon is incompletely elucidated. It has been suggested that the coexistence of a neuroendocrine phenotype in predominantly exocrine tumors correlates with overall prognosis3, 4 and seems of special importance in facilitating prostate cancer progression after androgen-deprivation therapy. Several mechanisms have been identified in this respect: neuroendocrine cells are androgen receptor negative, therefore they survive androgen deprivation; as an alternative, neuroendocrine cells produce peptides, hormones and growth factors that could stimulate the proliferation of exocrine prostate cancer cells and increase their aggressiveness through apoptosis inhibition and neoangiogenesis stimulation.5, 6, 7 Two recent studies in patients with hormone-refractory disease have demonstrated an independent poor prognostic role of elevated levels of circulating chromogranin A, a marker of neuroendocrine differentiation,8, 9 although in patients with hormone naive disease a significant relationship between amount of neuroendocrine cells and disease stage and grade but not with disease-free survival and overall survival has been unequivocally proven.10, 11, 12
Neuroendocrine differentiation regulatory mechanisms might play a role in the transition from an androgen-dependent to an androgen-resistant phenotype in prostate cancer, and in vivo studies, both in animals13 and humans,14 have shown that the neuroendocrine prostate cancer compartment increases after androgen deprivation.
Neuroendocrine cell development and differentiation is a complex and still poorly understood mechanism. Many experimental in vitro and in vivo models show that the evolutionarily conserved Notch signaling pathway negatively regulates a system of transcription factors, the basic helix-loop-helix (bHLH) family, during neuronal and endodermal endocrine mammalian development15, 16, 17 via a system of effectors, including hairy and enhancer of split 1 (Hes1), which repress bHLH-mediated transcription.18 Members of the bHLH family, like Math1, neurogenin 3 and neurogenic differentiation 1, have been demonstrated to regulate endocrine cell differentiation in the gastrointestinal tract and pancreas of mouse models. Among them, human achaete-scute homolog 1 (hASH1) plays a pivotal role in the development and differentiation of neuronal and endocrine cells, with special reference to foregut and midgut derivatives.19 With regard to human tumors of neuroendocrine lineage, hASH1 transcripts are known to be expressed in poorly differentiated neuroendocrine—small cell—carcinomas of the lung,20, 21, 22 gastrointestinal tract,23 as well as in neuroblastomas24 and medullary thyroid carcinomas,25 but not in well-differentiated endocrine tumors (carcinoids).21, 23 No data are currently available on the possible role of hASH1 in driving divergent neuroendocrine differentiation in non-neuroendocrine adenocarcinomas, such as prostate carcinoma, or in mixed endocrine–exocrine cancers. Recently, in a mouse model of prostate neuroendocrine carcinoma, exceptionally high expression levels of hASH1, in concert with a wide range of neuroendocrine markers including L-dopa decarboxylase, have been demonstrated.26 On the basis of such findings, hASH1 likely represents a critical actor to drive neuroendocrine phenotype development in prostate cancer: the present study was designed to analyze expression at both transcriptional and protein levels in a large series of prostatic cancers with various degrees of neuroendocrine differentiation, with and without androgen-deprivation therapy administration.
Materials and methods
Case selection
Group A: PC-NE/Untreated
To analyze hASH1 expression in prostate cancers with neuroendocrine differentiation, a series of 40 cases with neuroendocrine features at diagnosis, proven by chromogranin A immunohistochemistry, and without any previous treatment was collected.
Group B: PC-NonNE
To compare possible differential expression of hASH1 in non-neuroendocrine prostate cancers, a control group of previously untreated 25 prostate cancers showing no evidence of neuroendocrine differentiation by chromogranin A immunohistochemistry was entered in the study.
Group C: PC-NE/Treated
In addition, to investigate the possible effects of hormonal treatment in modulating hASH1 expression, a third group of 15 prostate cancers with neuroendocrine differentiation and previous androgen-deprivation therapy was also collected. All these patients were treated by endocrine therapy, consisting of androgen deprivation by LHRH (luteinizing hormone-releasing hormone) analog administration, for a period ranging from 6 to 86 months prior to sampling of the tissue specimen analyzed in this study.
Three additional cases of pure large cell neuroendocrine carcinomas primary of the prostate were also included in the study.
Total tissue material corresponded to 62 surgical samples and 21 needle biopsies or transuretral endoscopic resections. Formalin-fixed paraffin-embedded tissue material was available for histological review and further analysis. In 20 cases, fresh frozen tumor tissue (selected by frozen section examination of the corresponding tissue fragment) as well as parallel normal tissue, were also available. All tissue samples were anonymized by a staff member of the Pathology Department not involved in the study, and the study was approved by the Local Ethic Committee.
Immunohistochemistry
Five-micron-thick sections, serial to those used for conventional histopathological examination, were deparaffinized and then treated with graded alcohols and rehydrated in PBS pH 7.5. Endogenous peroxidase activity was blocked by absolute methanol and 0.3% hydrogen peroxide for 15 min. To assess the presence of neuroendocrine phenotype, a primary monoclonal antibody to chromogranin A (LK2H10, diluted 1:800; NeoMarkers, Fremont, CA, USA) was used. Human ASH1 protein was detected by means of a monoclonal antibody (24B72D11.1, diluted 1:150; BD Biosciences, San Jose, CA, USA). Antigen-retrieval procedure was performed for both chromogranin A and hASH1 by microwave heating (three 5 min passages at 750 W), in citrate buffer (pH 7.5) and EDTA buffer (pH 8.0) solutions, respectively. Immunoreactions were revealed by a dextran-chain (biotin-free) detection system (EnVision; DakoCytomation, Glostrup, Denmark), using 3,3′-diaminobenzidine (DAB; Dako) as a chromogen. A small cell lung carcinoma sample served as positive control for both antibodies. Negative control reactions were obtained by omitting the primary antibodies.
For both hASH1 and chromogranin A, the immune reaction was scored independently by two different researchers of the Pathology Department (IR and MV) as negative or positive using a semiquantitative three-tier system according to the percentage of reactive tumor cells (0: <5%; 1: 5–20%; 2: >20%).
Double immunohistochemical procedure
On selected normal and neoplastic prostate samples hASH1 and chromogranin A were investigated by double immunohistochemical reactions, using immunoperoxidase procedure for hASH1, followed by immunoalkaline phosphatase method Envision-AP (Dako) with Vector blue alkaline phosphatase substrate kit III (from Vector Laboratories, CA, USA) for chromogranin A, at the same conditions reported above. Moreover, to test the proliferative capability of hASH1-positive cells, double immunofluorescence experiments with hASH1 and Ki-67 antibodies were performed, as follows. After antigen-retrieval procedure by microwave heating in citrate buffer solution (pH 7.5), sections were incubated with rabbit monoclonal anti-Ki-67 (clone SP6; Lab Vision, Fremont, CA, USA) 1:200 overnight at 4°C, and then incubated with FITC-conjugated goat anti-rabbit immunoglobulin (Invitrogen Corporation, Carlsbad, CA, USA) diluted 1:200. Samples were then incubated with mouse anti-hASH1 diluted at 1:150, overnight at 4°C, followed by anti-mouse biotin-conjugated antibody (Dako) diluted 1:200 and detected with streptavidin Texas red-conjugated (Invitrogen). Nuclei were counterstained with 4′-6-diamidino-2-phenylindole (DAPI; Vysis Downers Grove, IL, USA) and immunostained slides were examined under a fluorescence microscope Olympus BX41 (Olympus Shinjuku Monolith, Tokyo, Japan) equipped with appropriate filters and analyzed by Soft Imaging System software.
RNA isolation from frozen tissues and qualitative RT-PCR of hASH1, Notch1 and Hes1
To screen hASH1, Notch1 and Hes1 RNA expression in prostate normal tissues and cancers, total RNA from snap-frozen normal and neoplastic tissues of 20 prostate cancer cases was extracted using QIAzol lysis Reagent (Qiagen, Tokyo, Japan), and cDNA was transcripted using 500 μg/ml oligodT (Roche Applied Science, Penzberg, Germany) and 500M-MLV RT (200 U/μl) (Invitrogen) according to standard protocols.
Expression of hASH1, Notch1 and Hes1 transcripts, were examined by qualitative real-time (RT)-PCR using primers and PCR conditions previously reported.23
RNA isolation from paraffin-embedded tissues and hASH1 quantitative real-time PCR
To parallel immunohistochemical data with hASH1 mRNA expression, 10 μm section, serial to those immunostained for chromogranin A, were cut in RNase-free conditions and stained with nuclear Fast Red (Sigma-Aldrich, St Luis, MO, USA) to highlight the tissue architecture. Under stereomicroscopic assistance, representative tumor areas positive and negative for chromogranin A in the parallel section, as well as normal peritumoral tissue, were identified, isolated by means of microdissection using a scalpel at a magnification of × 5–10, and analyzed separately. RNA isolation was performed by commercially available RNA extraction kits designed for paraffin material according to the manufacturer's instructions (High Pure RNA Paraffin Kit; Roche Applied Science, Milano, Italy). Relative cDNA quantification for hASH1 and an internal reference gene (β-actin) was performed using a fluorescence-based real-time detection method (ABI PRISM 7900 Sequence Detection System—Taqman; Applied Biosystems, Foster City, CA, USA). The sequences of the primers and probe used for β-actin have been previously published.27 Those used for hASH1 are listed in Table 1 and were designed according to the Ref Seq NM_004316 (http://www.ncbi.nlm.nih.gov/LocusLink). The PCR product size generated with these primers was 71 bp long and validated through gel electrophoresis and serial dilutions28 (Figure 1). The PCR mixture consisted of 1200 nmol/l of each primer, 200 nmol/l of the probe, 200 nmol/l each of dATP, dCTP, dGTP and dTTP, 3.5 mmol/l MgCl2 and 1 × Taqman Universal PCR Master mix to a final volume of 20 μl (all reagents were from Applied Biosystems). Cycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 46 cycles at 95°C for 15 s and 60°C for 1 min. Each measurement was performed in duplicate. To compare hASH1 expression in different tumors, the relative gene expression levels were expressed as ratios (differences between the Ct values) between two absolute measurements (genes of interest/internal reference gene). To test differences of hASH1 expression of different areas within individual tumors, the ΔΔCt values were calculated (subtracting ΔCt values of chromogranin A-positive and chromogranin A-negative tumor areas, analyzed separately), and converted to ratio by the following formula: 2−ΔΔCt.
Several runs with serial control cDNA dilutions were performed. The slope of the plot Ct vs log of input cDNA was −3.28 for hASH and −3.32 for β-actin (being −3.33 100% efficiency). The two efficiencies were comparable at a satisfactory level (<5%) with a correlation coefficient (R2) >0.98. Thus, the slope of the ΔCt values vs log of input cDNA was <0,1 (not shown).
Statistics
χ2 test was used to analyze the correlation between hASH1 protein expression and the clinicopathological characteristics, including age, Gleason score, tumor stage, PSA levels at the time of diagnosis, extent of neuroendocrine differentiation and therapy (see Table 2). t-Test was used to evaluate hASH1 mRNA expression levels in treated vs untreated patients. Statistical significance was set at P=0.05.
Results
hASH1 protein expression correlates with NE phenotype and antiandrogenic therapy administration
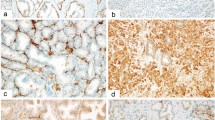
Human ASH1 nuclear protein was detected in scattered cells within normal and hyperplastic prostate glands. Double immunohistochemical procedure identified these cells as chromogranin A-positive neuroendocrine cells (Figure 2a). A strong and diffuse hASH1 expression was found in all three cases of pure primary large cell neuroendocrine prostate carcinomas (all scores 2) (Figure 2b). In adenocarcinomas, a strong correlation between hASH1 protein expression and the presence and extent of neuroendocrine phenotype was observed. In fact, it was absent in all prostate cancers lacking neuroendocrine features (PC-nonNE group) except for one case where nuclear hASH1 staining was observed in isolated tumor cells. By contrast, it was observed to a higher extent in therapy-naive prostate cancers showing neuroendocrine features (PC-NE/untreated group), with a strong correlation with the extent of chromogranin A-positive immunohistochemistry (P=0.02) (Figure 2c). By either double immunohistochemistry or comparison of parallel serial sections, hASH1 and chromogranin A distribution was localized in the same tumor areas. In addition, hASH1-positive immunohistochemistry was stronger (hASH1 score 2 cases) in tumor samples with more prominent chromogranin A reactive cells (score 2 cases), although this latter finding did not reach statistical significance (P=0.09). By means of double hASH1 and Ki-67 immunofluorescence, we could demonstrate that hASH1 was coexpressed in a fraction of proliferating tumor cells (Figure 2d). When comparing PC-NE/untreated with PC-NE/treated groups, a higher rate of hASH1-positive cases was observed in the latter group, (P=0.01). Human ASH1 protein expression was not correlated with any further clinicopathological parameter considered.
hASH1 nuclear protein was expressed in normal neuroendocrine cells of the prostate (a; double immunohistochemical procedure with chromogranin A, blue cytoplasmic and hASH1—brown nuclear), pure neuroendocrine carcinomas primary of the prostate (b) and in tumor areas of conventional adenocarcinomas with neuroendocrine differentiation (c), with partial coexpression with Ki-67 (d; hASH1 in red, Ki-67 in green and coexpression in yellow—bottom left) (a; double immunohistochemical procedure, immunoperoxidase and immunoalkaline phosphatase, × 1000; (b) left: H&E × 200, right: chromogranin A—top—and hASH1—bottom—immunostaining, × 200; (c) chromogranin A—left—and hASH1—right—immunostaining in the same tumor area, immunoperoxidase, × 200 and 400; (d) double immunofluorescence procedure, see text for details).
Constitutive hASH1, Notch1 and HES1 transcript expression in normal and neoplastic frozen prostate tissues
To screen transcript expression of Notch1, hASH1 and Hes1 in neoplastic prostate tissue, we tested by qualitative RT-PCR 20 tumors and the corresponding normal gland. Among them, five cases displayed on the corresponding paraffin tissue neuroendocrine features as represented by chromogranin A-positive immunohistochemistry. All 20 cases displayed detectable mRNA amounts of the three genes in both normal and neoplastic tissue specimens, without any correlation with the presence of the neuroendocrine phenotype. Therefore, we focused our further attention on hASH1 expression—which represent the final effector of the pathway—on paraffin-embedded specimens by means of quantitative RT-PCR, as detailed in the next paragraph.
High hASH1 mRNA expression levels in antiandrogen therapy-treated prostate cancers with NE features
To validate hASH1 immunohistochemical data and quantify transcript expression in chromogranin A-positive prostate cancers, all radical prostatectomy samples in groups PC-NE/untreated and PC-NE/treated were analyzed by RT-PCR from paraffin material, for a total of 18 cases. In 13 of them, chromogranin A-positive and chromogranin A-negative tumor areas from each individual case were analyzed separately, and the relative ratios compared according to the ΔΔCt method. A general upregulation of hASH1 transcripts in chromogranin A-positive as compared with chromogranin A-negative tumor cell populations was observed: 10 out of such 13 samples showed a ratio of hASH1 transcripts >twofold, which was considered significant according to the literature29 (Figure 3a). To test hASH1 mRNA expression in cases with or without previous androgen ablative therapy, a relative quantification using β-actin as the endogenous control and evaluating expression levels as ratio in a linearized scale was performed in 10 cases from PC-NE/untreated and 8 cases from PC-NE/treated groups. All values ranged from 0.1 to 17.7 (all unitless ratios). A higher hASH1 mRNA expression was observed in PC-NE/treated patients (1.6±6.9 and 0.6±2.8 in PC-NE/treated and PC-NE/untreated cases, respectively, P=0.01) (Figure 3b). Peritumoral normal prostate tissue showed low hASH1 expression levels (0.63±0.65), without relevant differences among samples from PC-NE/untreated and PC-NE/treated groups.
By RT-PCR, higher levels of hASH1 transcripts were detected in chromogranin A positive as compared with negative tumor cell populations (a; error bars represents s.d., which were calculated according to Applied Biosystems User Bullettin no. 2); higher levels of hASH1 transcripts were also detected in patients subjected to androgen-deprivation therapy (b; the line in the middle represents the median values, the boxes represents the range between 25th and 75th percentiles, and the whiskers represents the upper and lower values).
Discussion
An essential role of the Notch signaling pathway, and specifically of hASH1 transcription factor, has been reported in the development and control of biological activities of both normal and neoplastic neuroendocrine cells. Despite high expression levels of hASH1 have been demonstrated, among others, in poorly differentiated neuroendocrine carcinomas of the lung and gastrointestinal tract,21, 23 no data are currently available on its putative role in determining the occurrence of neuroendocrine differentiation in non-neuroendocrine tumors, a phenomenon that is still controversial from both pathogenetic and clinical sides. With special reference to prostate cancer, the presence of neuroendocrine features was proven to correlate with aggressive clinical behavior and hormone-refractory disease,8, 9, 30 and therefore a better knowledge of the mechanisms leading to this phenomenon would have major clinical impact. By qualitative and quantitative PCR and immunohistochemistry, we could identify a pivotal role of hASH1 molecule in the neuroendocrine differentiation process of prostate cancer, as it was associated to the presence of neuroendocrine features, with special regard to patients with previous androgen-deprivation hormonal treatment. These data are in agreement with a single study published by other investigators,26 in which the expression of the mouse homolog of hASH1 was detected in neuroendocrine cancers derived from CR2-Tag transgenic mice prostate model, and in a limited number of human prostate carcinoma samples.
Both immunohistochemistry and quantitative PCR data topographically ‘localized’ hASH1 molecule in the same areas showing neuroendocrine features. Such observation outlines a patchy expression of hASH1 in prostate cancer, as the result of still unrecognized regulatory mechanisms that act heterogeneously in those prostate cancer cell populations displaying neuroendocrine phenotype, and probably reflect the acquisition of a specific phenotype in prostate cancer cells in which hASH1 expression most likely represents a peculiar functional status. In this respect, the higher prevalence of hASH1 expression at both transcriptional and protein levels in prostate cancer patients previously submitted to androgen ablation therapy suggests that hASH1 expression in prostate cancer (and possibly normal) cells is under hormonal control. Although functional in vivo studies are needed to better clarify this aspect, some literature data may help to draw a possible scenario. The Notch pathway is involved in prostate development and differentiation processes, and Notch itself is expressed in progenitor basal cells of the prostate with a positive modulation by androgen deprivation in transgenic mouse model.31 Since in physiologic conditions, Notch negatively modulates hASH1 expression, this latter finding is apparently in contrast with our data. However, recent reports aimed to define possible mechanisms leading to hormone resistance in prostate cancer identified specific alterations (cytoplasmic vs nuclear localization) of Notch modulators in prostate cancer, namely Hey1, which acts as corepressor of androgen receptor-mediated signaling and represses bHLH transcription factors, such as hASH1.32 Abnormalities of Notch itself or Notch-positive effectors might be responsible for both the development of androgen-independent prostate cancers as well as of the de-repression of hASH1 transcriptional activities with the development of neuroendocrine-differentiated tumor clones. In this respect, our data on the lack of correlation between Notch1 and Hes1 and the presence of neuroendocrine phenotype in prostate tissues is inconclusive, and more detailed experiments are needed to clarify the activation status of such molecules in prostate cancer with neuroendocrine differentiation.
The functional activity of hASH1 in cell growth control is therefore still largely unknown, possibly varying from transcriptional regulation of cell-cycle-related molecules to auto/paracrine signaling of growth factors. In neuroendocrine prostate cancer cells derived from CT2-TAg transgenic mice, RNA interference studies demonstrated the capability of the mouse hASH1 homolog to negatively regulate cell cycle (ie, promoting cyclin-dependent kinase inhibitors expression) and to increase cAMP signaling pathways as a mechanism to promote neuroendocrine differentiation.26 By contrast, the same methodological approach in neuroendocrine lung cancer model demonstrated that hASH1 inhibition led to cell-cycle arrest and apoptotic cell death.22
In conclusion, our data suggest hASH1 as a critical factor involved in neuroendocrine differentiation in prostate cancer, with special reference to patients treated with androgen-deprivation therapy. These findings are relevant to better characterize the mechanisms that induce and maintain the neuroendocrine phenotype in prostate cancer, which represent an independent poor prognostic indicator and is a major feature responsible for hormone-refractory disease. Future hASH1 functional studies will highlight the potential impact of new therapeutic strategies targeted to the Notch pathway (ie, γ-secretase inhibitors) on growth control and restoration of androgen sensitivity in currently uncurable neuroendocrine-differentiated prostate cancers.
References
Cooperberg MR, Moul JW, Carroll PR . The changing face of prostate cancer. J Clin Oncol 2005;23:8146–8151.
Abrahamsson PA . Neuroendocrine cells in tumor growth of the prostate. Endocr Relat Cancer 1999;6:503–519.
Tricoli JV, Schoenfeldt M, Conley BA . Detection of prostate cancer and predicting progression: current and future diagnostic markers. Clin Cancer Res 2004;10:3943–3953.
di Sant'Agnese PA . Neuroendocrine differentiation in prostatic carcinoma: an update on recent developments. Ann Oncol 2001;12 (Suppl 2):S135–S140.
Bonkhoff H . Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol 2001;12 (Suppl 2):S141–S144.
Xing N, Qian J, Bostwick D, et al. Neuroendocrine cells in human prostate over-express the anti-apoptosis protein survivin. Prostate 2001;48:7–15.
Mazzucchelli R, Montironi R, Santinelli A, et al. Vascular endothelial growth factor expression and capillary architecture in high-grade PIN and prostate cancer in untreated and androgen-ablated patients. Prostate 2000;45:72–79.
Berruti A, Mosca A, Tucci M, et al. Independent prognostic role of circulating chromogranin A in prostate cancer patients with hormone-refractory disease. Endocr Relat Cancer 2005;12:109–117.
Taplin ME, George DJ, Halabi S, et al. Prognostic significance of plasma chromogranin A levels in patients with hormone-refractory prostate cancer treated in Cancer and Leukemia Group B 9480 study. Urology 2005;66:386–391.
Bollito E, Berruti A, Bellina M, et al. Relationship between neuroendocrine features and prognostic parameters in human prostate adenocarcinoma. Ann Oncol 2001;12 (Suppl 2):S159–S164.
Puccetti L, Supuran CT, Fasolo PP, et al. Skewing towards neuroendocrine phenotype in high grade or high stage androgen-responsive primary prostate cancer. Eur Urol 2005;48:215–221.
Berruti A, Mosca A, Porpiglia F, et al. Chromogranin A expression in patients with hormone naive prostate cancer predicts the development of hormone refractory disease. J Urol 2007;178:838–843.
Jongsma J, Oomen MH, Noordzij MA, et al. Different profiles of neuroendocrine cell differentiation evolve in the PC-310 human prostate cancer model during long-term androgen deprivation. Prostate 2002;50:203–215.
Sciarra A, Monti S, Gentile V, et al. Variation in chromogranin A serum levels during intermittent vs continuous androgen deprivation therapy for prostate adenocarcinoma. Prostate 2003;55:168–179.
Naya FJ, Huang HP, Qiu Y, et al. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev 1997;11:2323–2334.
Gradwohl G, Dierich A, LeMeur M, et al. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA 2000;97:1607–1611.
Yang Q, Bermingham NA, Finegold MJ, et al. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 2001;294:2155–2158.
Jensen J, Pedersen EE, Galante P, et al. Control of endodermal endocrine development by Hes-1. Nat Genet 2000;24:36–44.
Guillemot F, Lo LC, Johnson JE, et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 1993;75:463–476.
Borges M, Linnoila RI, van de Velde HJ, et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 1997;386:852–855.
Jiang SX, Kameya T, Asamura H, et al. hASH1 expression is closely correlated with endocrine phenotype and differentiation extent in pulmonary neuroendocrine tumors. Mod Pathol 2004;17:222–229.
Osada H, Tatematsu Y, Yatabe Y, et al. ASH1 gene is a specific therapeutic target for lung cancers with neuroendocrine features. Cancer Res 2005;65:10680–10685.
Shida T, Furuya M, Nikaido T, et al. Aberrant expression of human achaete-scute homologue gene 1 in the gastrointestinal euroendocrine carcinomas. Clin Cancer Res 2005;11:450–458.
Rostomily RC, Bermingham-McDonogh O, Berger MS, et al. Expression of neurogenic basic helix-loop-helix genes in primitive neuroectodermal tumors. Cancer Res 1997;57:3526–3531.
Ball DW, Azzoli CG, Baylin SB, et al. Identification of a human achaete-scute homolog highly expressed in neuroendocrine tumors. Proc Natl Acad Sci USA 1993;90:5648–5652.
Hu Y, Ippolito JE, Garabedian EM, et al. Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice. J Biol Chem 2002;277:44462–44474.
Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006;107:1589–1596.
Taron M, Rosell R, Felip E, et al. BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet 2004;13:2443–2449.
Qin L, Qiu P, Wang L, et al. Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J Biol Chem 2003;278:19723–19731.
Feldman BJ, Feldman D . The development of androgen-independent prostate cancer. Nat Rev Cancer 2001;1:34–45.
Wang Z, Zhang Y, Li Y, et al. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther 2006;5:483–493.
Belandia B, Powell SM, Garcia-Pedrero JM, et al. Hey1, a mediator of notch signaling, is an androgen receptor corepressor. Mol Cell Biol 2005;25:1425–1436.
Acknowledgements
This work was supported by grants from the Italian Ministry of University (ex 60% to MP and MV).
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
All authors declare the absence of any financial and personal relationships between themselves and others that might bias their work and of any potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Rapa, I., Ceppi, P., Bollito, E. et al. Human ASH1 expression in prostate cancer with neuroendocrine differentiation. Mod Pathol 21, 700–707 (2008). https://doi.org/10.1038/modpathol.2008.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2008.39
Keywords
This article is cited by
-
hASH1 nuclear localization persists in neuroendocrine transdifferentiated prostate cancer cells, even upon reintroduction of androgen
Scientific Reports (2019)
-
EZH2 promotes progression of small cell lung cancer by suppressing the TGF-β-Smad-ASCL1 pathway
Cell Discovery (2015)
-
Neuroendocrine carcinoma of the esophagus: clinicopathologic study of 10 cases and verification of the diagnostic utility of mASH1, NeuroD1, and PGP9.5
Esophagus (2014)
-
DNA copy number alterations and PPARG amplification in a patient with multifocal bladder urothelial carcinoma
BMC Research Notes (2012)
-
Human achaete-scute homolog-1 expression in neuroendocrine breast carcinoma
Virchows Archiv (2012)