Abstract
Urovysion™ fluorescence in situ hybridization (FISH) is a sensitive and specific test used to diagnose urothelial carcinoma in urine. It detects aneuploidy of chromosomes 3, 7 and 17, and loss of both 9p21 loci in malignant urothelial cells. We evaluated Urovysion™ FISH in non-urothelial carcinoma involving bladder to determine its possible application to their diagnosis and surveillance. Paraffin blocks from 31 non-urothelial bladder carcinomas, 12 pure urothelial carcinomas and 2 urothelial carcinomas with squamous differentiation were tested according to Vysis-Abbot Laboratories' recommended standards. Cases included 15 primary squamous carcinoma, 2 urothelial carcinoma with squamous differentiation, 4 primary adenocarcinoma, 5 colonic, 4 prostatic and 1 cervical adenocarcinoma. Total 60% of squamous, 83% of pure urothelial, 100% of urothelial carcinoma with squamous differentiation and 100% of primary and secondary adenocarcinomas hybridized successfully; 2/10 (11%) squamous carcinomas and 11/14 (79%) primary and secondary adenocarcinomas were Urovysion™ FISH-positive with primary adenocarcinomas accounting for 75% (3/4), colonic, 80% (4/5), prostatic, 75% (3/4) and cervical, 100% (1/1) positivity. Total 70% (7/10) of pure urothelial carcinomas and 100% (2/2) of urothelial carcinomas with squamous differentiation were Urovysion™ FISH-positive. In conclusion, we found that chromosomal abnormalities tested for by Urovysion™ FISH may be seen in non-urothelial carcinomas of bladder. These false-positive results were frequent in primary and secondary adenocarcinoma and rare in squamous carcinoma. This has significant implications for the accurate diagnosis and management of patients with urinary tract cancer. Urovysion™ FISH cannot be used to definitively diagnose squamous carcinoma or adenocarcinoma nor can it be used to differentiate the two from urothelial carcinoma. However, it may be useful as a surveillance tool in established primary and secondary bladder adenocarcinoma. Cytopathologists and urologists should correlate Urovysion™ FISH results with cytomorphology and clinical information.
Similar content being viewed by others
Main
Urothelial carcinoma is the seventh most common carcinoma worldwide. It accounts for 90–95% of bladder cancers in the Western hemisphere and has a sixfold higher incidence in developed countries and Europe when compared with developing nations.1 In the United States alone, the number of new cases diagnosed in 2007 was 120 400 with 27 340 disease-related deaths in the same year.2 Invasive urothelial carcinoma is defined by the World Health Organization (WHO) as urothelial cancer that has invaded the lamina propria of the bladder.1 Although the majority of tumors arise in the bladder, the renal pelvis and ureter are also, albeit less frequently, involved.3 There are several established risk factors for urothelial carcinoma. These include occupational exposure to the aromatic amines benzidine and 1-/2-naphthylamine, tobacco smoking, cyclophosphamide, arsenic, analgesics and bladder irritants such as chronic urinary tract infections and stones.1, 3 The entire urothelium is exposed to many of these carcinogens and as a result tumors may arise at any point along the urinary tract.3 Radiation therapy of neighboring organs is another potential risk factor that has been linked to bladder carcinoma.3
Non-urothelial bladder tumors have also been described and these account for fewer than 10% of bladder tumors. They include squamous-cell carcinoma, primary and secondary adenocarcinoma and neuroendocrine tumors. Squamous carcinoma of the bladder is a rare, highly aggressive tumor that comprises less than 5% of bladder cancers in the United States.4 In the Western hemisphere, the most common risk factors for squamous carcinoma are non-bilharzial. These include keratinizing squamous metaplasia, non-invasive squamous lesions, smoking and chronic mucosal irritants such as calculi and indwelling catheters.4, 5, 6 In Africa, however, chronic infection with Schistosoma hematobium (bilharziasis) is the most common cause.4, 6 Although these tumors are derived from urothelium, they show pure squamous morphology and at the time of diagnosis have often infiltrated the muscularis propria and are thus associated with a poor clinical outcome.4, 6 Primary adenocarcinoma of the bladder shows pure glandular morphology. It is extremely rare and accounts for less than 2% of all malignant bladder tumors. Risk factors include intestinal metaplasia, bladder obstruction and chronic bladder irritation.7 Although primary adenocarcinoma of bladder is a well-known entity, the more frequent cause of bladder adenocarcinoma is direct extension from adjacent pelvic tumors or metastases from distal sites. Colonic carcinoma accounts for the majority of these cases, followed by prostatic, endometrial and cervical adenocarcinoma.7
The gold standard for diagnosis and surveillance of urothelial carcinoma has traditionally been cystoscopy and urine cytology, however both have limitations.8, 9, 10, 11, 12, 13, 14 Although urine cytology is excellent for detecting high-grade urothelial carcinoma (sensitivity and specificity >75%), it has a low sensitivity (20–60%) for detecting low-grade tumors.8, 10, 11, 12, 13, 14 The low sensitivity of urine cytology, the invasiveness of cystoscopy and its limited usefulness in detecting flat and inaccessible lesions have prompted increased demand for newer, more sensitive and non-invasive tests for detection of urothelial carcinoma. This has led to the development of new sophisticated molecular techniques that have a higher sensitivity and specificity for urothelial carcinoma detection.12, 15 One such technique is the now commercially available multicolor, multitarget Urovysion™ fluorescence in situ hybridization (Urovysion™ FISH) assay that was created by Vysis Incorporated (Vysis-Abbot Laboratories, Downers Grove, IL, USA).16 Urovysion™ FISH is the first molecular test that uses DNA probes to identify the most common urothelial carcinoma-related chromosomal abnormalities in urine. The Food and Drug Administration initially approved its use as a surveillance tool for patients with a history of urothelial carcinoma. However, this was later extended to include its use as a screening tool in patients with hematuria and risk factors for urothelial carcinoma.17, 18 Urovysion™ FISH has a reported sensitivity of 73–92%, a specificity of 89–96% for urothelial carcinoma detection and several studies have confirmed its usefulness in the diagnosis and surveillance of these tumors.8, 14, 19, 20, 21, 22 The test is designed to detect aneuploidy for chromosomes 3, 7, 17, and loss of the 9p21 locus in malignant urothelial cells that are shed in the urine of persons with urothelial carcinoma. These represent some of the most common chromosomal abnormalities in urothelial carcinoma. Polysomy of chromosomes 3, 7 and 17 are associated with high-grade urothelial carcinoma and deletions of chromosome 9p21 (where the p16INK4a tumor suppressor gene resides) are seen in up to 60% of superficial low-grade, papillary tumors.22, 23, 24 Although Urovysion™ FISH was initially approved for use in urine samples, it can also be applied to paraffin-embedded tissues.
We recently had three cases of non-urothelial bladder tumors with positive Urovysion™ FISH results in corresponding urine samples. These included squamous carcinoma (n=1) and metastatic colonic adenocarcinoma of the bladder (n=2). In addition, the adenocarcinoma cases had cytologic features that were initially felt to be consistent with urothelial carcinoma. The cytomorphology combined with the positive Urovysion™ FISH results led to the erroneous diagnosis of urothelial carcinoma. It was only after confirmatory bladder biopsy was performed that the correct diagnosis of colonic adenocarcinoma was rendered. These index cases prompted us to ask whether non-urothelial tumors such as squamous carcinoma and adenocarcinoma could cause a false positive Urovysion™ FISH result, and if so, could the test be used as a diagnostic and/or surveillance tool for non-urothelial carcinoma.
Materials and methods
The Pathology files at the Medical College of Georgia and the Charlie Norwood Veterans Affairs Medical Center were searched for cases of squamous carcinoma and adenocarcinoma of bladder that were diagnosed between the years 2000 and 2007. The study was approved by the institutional review boards of both institutions and was in compliance with HIPAA regulations. Hematoxylin and eosin-stained slides (as well as immunohistochemical slides, where applicable) from formalin-fixed, paraffin-embedded tissues were reviewed by two reference pathologists and a diagnosis was rendered in each case using WHO criteria. A representative paraffin block containing ≥80% tumor cells was then selected for Urovysion™ FISH testing and the target area for hybridization was highlighted on each representative slide. All metastatic adenocarcinomas were confirmed either by immunohistochemistry, and/or review of original primary tumors. Twelve cases of pure urothelial carcinoma were run concurrently as controls.
Fluorescence In Situ Hybridization
The Urovysion™ FISH probe mixture consisted of probes directed against the peri-centromeric regions of chromosomes 3 (CEP3), 7(CEP7) and 17 (CEP17), and band 9p21 locus (LSI 9p21). The probes were labeled with Texas red (CEP3), spectrum green (CEP7), spectrum aqua (CEP17) and spectrum gold fluorophores (LSI 9p21). Paraffin-embedded sections were initially baked overnight at 56°C then deparaffinized through multiple rinses in Heme De. Slides were dehydrated for 5 min in 100% ethanol at room temperature (RT) and then immersed in pretreatment solution at 80°C for 10 min. They were then washed in purified water for 3 min and then incubated in a protease buffer at 37°C for 15 min. Following this, the slides were immersed in purified water for 3 min, 10% neutral buffered formalin at RT for 10 min, then washed in purified water and allowed to air dry. They were then run through three successive ethanol washes of 70, 85 and 100% concentration. The Urovysion™ probe solution was then added to the target area on the slides and a cover slip was placed. The slides and probes were codenatured at 75°C for 2 min then placed in a hybridization chamber overnight at 37°C. Rubber cement was then used to seal the slides. Following hybridization unbound probes were washed and nuclei were counterstained with DAPI stain, which allowed the tumor nuclei to fluoresce bright blue. A fluorescence microscope equipped with appropriate excitation and emission filters was then used to visualize the intense red, green, gold and aqua fluorescent signals. Urovysion™ probe signals and DAPI counter-stains were viewed using the following filters: DAPI single bandpass, aqua single bandpass (chromosome 17), gold single bandpass (chromosome 9p21) and red/green dual bandpass (chromosomes 3 and 7). Positive and negative controls were run concomitantly to monitor assay performance and accuracy of signal viewing.
A cell was determined to be abnormal if there were more than two signals for chromosomes 3 (red), 7 (green) or 17 (aqua) or, a loss of both copies of LSI 9p21 (gold). A minimum of 25 tumor cells were visualized and evaluated for these chromosomal changes. If no abnormalities were detected then the remaining cells were counted until a sufficient number of cells with chromosomal abnormalities was found or until 200 cells were evaluated. A positive result was the presence of ≥4 (or >10%) cells with gains of 2 or more of chromosomes 3, 7 and 17. In the case of chromosome 9 a positive result was one in which ≥12 cells showed zero 9p21 signals.
Results
A total of 29 cases of non-urothelial carcinoma of the bladder, 2 cases of urothelial carcinoma with squamous differentiation and 12 cases of pure urothelial carcinoma of the bladder were selected, for a total of 43 cases. Each non-urothelial carcinoma was classified according to WHO criteria as primary pure squamous carcinoma (n=15) (Figure 1), urothelial carcinoma with squamous differentiation (n=2) and primary or secondary adenocarcinoma (n=14). The adenocarcinoma cases included primary pure bladder adenocarcinoma (n=4) and secondary or locally invasive adenocarcinoma (n=10) (Figure 2). Secondary adenocarcinomas included tumors extending from the colon (n=5), prostate (n=4) and cervix (n=1). Of all 43 cases, 35 (81%) hybridized (25/35 non-urothelial carcinomas (71%) and 10/12 urothelial carcinomas (29%)). Tables 1, 2 and 3 summarize the results of Urovysion™ FISH.
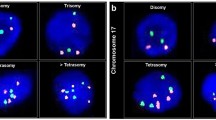
(a) Invasive keratinizing squamous-cell carcinoma of bladder. Note preserved normal urothelium on the left and invasive tumor on the right (hematoxylin and eosin, × 200). (b–d) Positive Urovysion™ FISH result with green (chromosome 7), red (chromosome 3), gold (chromosome 9p21) and aqua (chromosome 17) probes. Squamous cell carcinoma with multiple copies of (b) chromosomes 3 and 17, (c) chromosomes 7 and 17 and (d) chromosomes 3, 7 and 17.
Bladder wall involved by (a) colonic adenocarcinoma (hematoxylin and eosin, × 100), (b) primary mucinous adenocarcinoma (hematoxylin and eosin, × 200), (c) cervical adenocarcinoma (hematoxylin and eosin, × 100) and (d) prostatic adenocarcinoma, focally invading the muscularis propria (hematoxylin and eosin, × 200).
Of the 15 pure squamous carcinomas, only 11/15 (73%) specimens hybridized. Two of the 11 (18%) was Urovysion™ FISH-positive and 9/11 (82%) were Urovysion™ FISH-negative (Figure 1). The two positive squamous carcinoma cases showed polysomy of chromosomes 3 and 17 in one case and polysomy of chromosomes 3, 7 and 17 in the other (Tables 2 and 3). Four of 15 cases (27%) failed to hybridize and all of these blocks were greater than 5 years old. The most common polysomy in the squamous carcinoma cases was polysomy of chromosomes 3 and 17 (2/2 cases (100%)), followed by polysomy of chromosome 7 (1/2 cases (50%)) (Tables 2 and 3). Loss of the 9p21 allele was not observed (Figures 3 and 4).
Urovysion™ FISH with green (chromosome 7), red (chromosome 3), gold (chromosome 9) and aqua (chromosome 17) probes. (a) Primary mucinous adenocarcinoma of bladder with multiple copies of chromosome 3, 7 and 17. (b) Prostatic adenocarcinoma with multiple copies of chromosomes 3, 7, 9 and 17. (c) Cervical adenocarcinoma with multiple copies of chromosome 3. (d) Colonic adenocarcinoma with multiple copies of chromosomes 3, 7 and 17.
Of all the adenocarcinomas, 11/14 (79%) were Urovysion™ FISH-positive and 3/14 (21%) were negative. Of the primary bladder adenocarcinomas, 3/4 (75%) were Urovysion™ FISH-positive and 1/4 (25%) was negative (Table 1). The three positive primary adenocarcinomas showed polysomy of 3 and 17, polysomy of chromosomes 9 and 17, and polysomy of chromosomes 3, 7 and 9, respectively (Tables 2 and 3). Polysomy of chromosome 9 was not interpreted as a positive result however because the Urovysion™ FISH assay requires loss of both 9p21 alleles in order to consider a result positive. Of the colonic carcinomas 4/5 (80%) were Urovysion™ FISH-positive and 1/5 (20%) was negative. Three of 4 (75%) positive cases showed polysomy of chromosome 3, and 1/4 (25%) showed polysomy of chromosomes 3, 7 and 17. Of the prostatic carcinomas 3/4 (75%) were Urovysion™ FISH-positive and 1/4 (25%) was negative. One positive prostatic adenocarcinoma showed polysomy of chromosome 3, one showed polysomy of chromosomes 3, 7, 9 and 17 and one showed polysomy of chromosome 17. One cervical carcinoma was Urovysion™ FISH-positive and showed polysomy of chromosome 3. The most common chromosomal abnormality noted in all adenocarcinomas was polysomy of chromosome 3 (9/11 cases (82%)), followed by polysomy of chromosome 17 (5/11 cases (45%)). This was regardless of tumor type and degree of differentiation. The least common chromosomal abnormalities were polysomy of chromosomes 7 (3/11 cases (27%)) and chromosome 9 (3/11 cases (27%)). Loss of the 9p21 allele was not observed in any of the adenocarcinoma cases.
Of the 12 pure urothelial carcinomas, 2/12 (17%) failed hybridization. A total of 7 of the remaining 10 (70%) were Urovysion™ FISH-positive and 3/10 (30%) were negative (Tables 2 and 3). The 7 positive cases showed polysomy of chromosome 3 (1/7 cases (14%)), polysomy of chromosomes 3, 7 and 17 (2/7 cases (29%)), polysomy of chromosomes 3 and 17 (3/7 cases (43%)) and polysomy of chromosomes 7 and 17 (1/7 cases (14%)) (Tables 2 and 3). The most common polysomy noted in urothelial carcinoma was polysomy of chromosome 3 (6/7 cases (86%)) and 17 (6/7 cases (86%)). Polysomy of chromosome 7 (3/7 cases (43%)) was the least common chromosomal abnormality among the urothelial carcinomas (Table 3). Both cases of urothelial carcinoma with squamous differentiation (100%) were Urovysion™ FISH-positive and showed polysomy of chromosomes 3 and 17 in one case, and polysomy of chromosomes 7 and 17 in the other (Tables 2 and 3).
Discussion
Urothelial carcinoma is a primary urinary collecting system cancer that is derived from urothelium. Owing to the exposure of the entire urothelium to most of the inciting factors, tumor may arise anywhere along the collecting system and may also seed the urothelium proximal and distal to the main tumor. Studies have shown that there are numerous reproducible molecular abnormalities that occur during the development and progression of urothelial carcinoma.3, 23, 24 When normal urothelium undergoes mutations of chromosome 9p and fibroblast growth factor receptor 3, this may lead to the development of urothelial hyperplasia or low-grade papillary urothelial carcinoma.3 Up to 70% of low-grade tumors recur, however they may also transform into high-grade invasive urothelial carcinoma. High-grade tumors, including in situ urothelial carcinoma may also arise de novo. Numerous studies have shown that high-grade urothelial carcinoma and in situ urothelial carcinoma show genetic alterations that are distinct from those seen in their low-grade counterparts. These often involve the retinoblastoma, p53, RAS, epidermal growth factor receptor and HER2/NEU genes, which are present on chromosomes 13q14, 17p13, 3p21, 7p12 and 17p21, respectively. These genetic abnormalities have been identified in as few as 35%, and as many as 100% of high-grade noninvasive and invasive urothelial carcinomas. Recent studies have focused on identifying these DNA-related changes in urine samples, as they often precede the appearance of macroscopic and microscopic lesions, thereby allowing detection of subclinical disease.9, 25 Urovysion™ FISH is one such molecular test that has been shown to have high sensitivity and specificity for the detection of urothelial carcinoma in urine.8, 14, 19, 21, 26
We recently observed several cases of non-urothelial carcinoma that were Urovysion™ FISH-positive. Others have also observed these coincidental overlaps with non-urothelial tumors. Yoder et al in his study of Urovysion™ FISH in bladder cancer surveillance found five cases of non-urothelial carcinoma with positive results.27 Their cases included primary bladder adenocarcinoma (n=2), small-cell carcinoma (n=2) and squamous carcinoma (n=1) of bladder. Urovysion™ FISH was originally created for the diagnosis of urothelial carcinoma and was not intended for the diagnosis of non-urothelial cancers. Its usefulness in these tumors therefore has, to the best of our knowledge, not been investigated and its use is currently not the standard of practice. Despite this fact some urologists are using Urovysion™ FISH to monitor some patients with primary non-urothelial tumors (the authors' personal observation).
In our study we noted that the characteristic chromosomal abnormalities tested for by Urovysion™ FISH were sometimes, albeit infrequently, observed in squamous carcinoma of the bladder. Classical molecular and cytogenetic analyses have shown that the chromosomal abnormalities seen in squamous carcinoma of bladder are somewhat similar to those tested for by the Urovysion™ FISH assay.4 In FISH studies conducted by Pycha et al up to 78% of squamous carcinoma of bladder had trisomy of chromosomes 7 and 17, both of which could potentially cause a positive Urovysion™ FISH result.28, 29 In our study only two squamous carcinomas were Urovysion™ FISH positive and both of these cases showed polysomy of chromosome 17. Polysomy of chromosome 7 was also observed in one of these cases. It would then appear that although Urovysion™ FISH may occasionally be positive in squamous carcinoma, this positivity is uncommon and so the test is not useful for the diagnosis or surveillance of squamous carcinoma of bladder. Because the neoplastic cells of squamous carcinoma tend to show typical cytologic features such as keratinized spindle, ‘fiber’, ‘kite’ and ‘tadpole’ cells a positive Urovysion™ FISH result in such cases may be less likely to lead to an erroneous diagnosis of urothelial carcinoma. We noted Urovysion™ FISH positivity in both cases of urothelial carcinoma with squamous differentiation. In both of these cases, we made a deliberate attempt to select blocks that had a pure squamous population and despite this both cases were still Urovysion™ FISH-positive. These results suggest that in cases where one is attempting to distinguish pure squamous carcinoma from urothelial carcinoma with squamous differentiation, the Urovysion™ FISH assay may prove helpful, in that a positive result would favor the latter rather than the former.
The majority of the adenocarcinomas tested were Urovysion™ FISH-positive. Colonic adenocarcinoma showed the highest positivity followed by prostatic and primary bladder adenocarcinoma. Few studies have examined the chromosomal abnormalities in primary bladder adenocarcinoma. Some of these have shown loss of chromosomes 9q (17% of cases), 17p (50% of cases), 8p (50% of cases), 11p (43% of cases) and 9p (50% of cases).7 However, none of these chromosomal abnormalities are apt to cause a positive Urovysion™ FISH result. In our cases of pure primary adenocarcinoma, we observed polysomy of all four chromosomes (chromosomes 3, 7, 9 and 17) and no deletions. In advanced colonic carcinoma, monosomy of chromosome 18 as well as polysomy of chromosomes 7 and 17 have been reported and the latter two abnormalities would cause a positive Urovysion™ FISH result.30, 31 In our study, the most common chromosomal abnormality observed in colonic adenocarcinoma was polysomy of chromosome 3. Polysomy of chromosome 7 was only observed in one case. Although primary bladder adenocarcinoma does not have any specific distinguishing features on urine cytology, colonic carcinoma has several suggestive cytologic features. These include tall columnar cells with overlapping, hyperchromatic nuclei and abundant background necrosis. These features, although suggestive of colon cancer, are by no means diagnostic and if one is not highly suspicious of this differential one could easily misinterpret these cells as being urothelial in origin, as evidenced by one of our index cases. In addition, fluid cytology may cause some distortion or ‘rounding up’ of the neoplastic columnar cells, thereby affecting their distinction from urothelial carcinoma. Prostatic adenocarcinoma shares some chromosomal abnormalities with urothelial carcinoma in that it may also show gains of chromosomes 7 and 17,32 which could cause a positive Urovysion™ FISH result. In particular, gains of chromosome 7 are seen in locally advanced and/or metastatic prostatic adenocarcinoma.33 The neoplastic cells of prostatic adenocarcinoma are only rarely shed in urine and more often than not these tumors are Gleason score 8 or greater. These tumor cells are small and relatively bland, with moderate amounts of cytoplasm and a single prominent nucleolus.34 These cytologic features are not specific for prostatic cancer, and so, when combined with a positive Urovysion™ FISH result may lead to misdiagnosis as urothelial carcinoma.
Because the Urovysion™ FISH assay was initially created for the diagnosis of urothelial carcinoma, the positive results in these non-urothelial carcinoma cases were interpreted as being ‘false’-positives. This has significant implications for the accurate diagnosis, monitoring and management of patients with urinary tract cancer. Misclassification of tumors can lead to delayed diagnosis and unnecessary or inappropriate surgery or chemo-radiation, both of which are associated with significant morbidity and mortality. A potential benefit of a ‘false-positive’ result however is that it may be the first indication of the presence of a non-urothelial tumor. It may also assist in identifying locally advanced colonic or prostatic tumors in patients with subclinical disease.
Limitations of our study include the small study size and the fact that some samples failed to hybridize. Several factors may impair hybridization efficiency. These include the thickness and degree of fixation of the tissues, cautery artifact and the age of the paraffin blocks. We noted that samples that were extensively cauterized or greater than 5 years old did not hybridize. Unlike urine samples paraffin blocks have additional non-urothelial elements including stromal cells and inflammatory cells which also stain with DAPI counterstain. The cells of interest may also overlap each other or be covered by these non-urothelial elements thus limiting morphologic evaluation and reading of fluorescent signals. We attempted to counter this potential problem by only selecting slides that had 80% or greater tumor cells and limited non-urothelial tissue. In addition, two reference pathologists selected and highlighted target areas on the corresponding hematoxylin and eosin slides before the paraffin blocks were hybridized as these were felt to be most representative of the tumors. In addition, we reviewed all available slides on each case to ensure that only those with pure squamous or glandular morphology were selected when appropriate.
In conclusion, while Urovysion™ FISH is indisputably one of the most significant breakthroughs in the diagnosis and monitoring of urothelial carcinoma, our study highlights its limitations. Positive FISH results may be seen in non-urothelial tumors including adenocarcinoma and squamous carcinoma. These false positive results are fairly uncommon in squamous carcinoma but are frequent in primary and secondary adenocarcinoma of the bladder. Cytopathologists and urologists alike should be aware of this potential pitfall. In patients with a known history of colonic, prostatic and gynecologic adenocarcinoma Urovysion™ FISH results should be interpreted with caution particularly in those with locally aggressive or advanced disease. A more judicious practice for cytopathologists might be one where FISH results are routinely evaluated in conjunction with urine cytology samples, as well as clinical and radiologic information. Because of the rarity of a false-positive result in squamous carcinoma, Urovysion™ FISH is not recommended for monitoring patients with a history of squamous-cell carcinoma of bladder. However, as primary and secondary bladder adenocarcinomas show significant overlapping chromosomal aberrations with urothelial carcinoma, Urovysion™ FISH cannot reliably distinguish these two tumor types, nor can it be used to definitively diagnose adenocarcinoma of bladder. Urovysion™ FISH does however appear to have some, albeit limited, usefulness as a surveillance tool in patients with established primary and secondary adenocarcinoma of bladder. It will be necessary to conduct larger comparative studies on urine cytology specimens to confirm the clinical usefulness of Urovysion™ FISH in non-urothelial carcinomas of the bladder.
References
Lopez-Beltran A, Sauter G, Gasser T, et al. Infiltrating urothelial carcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital System, 1st edn. IARC Press: Lyon, France, 2004, pp 93–109.
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66.
Kakizoe T . Development and progression of urothelial carcinoma. Cancer Sci 2006;97:821–828.
Grignon DJ, El-Bolkainy M, Schimitz-Drager BJ, et al. Squamous cell carcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital System, 1st edn. IARC Press: Lyon, France, 2004, pp 124–129.
Guo CC, Fine SW, Epstein JI . Noninvasive squamous lesions in the urinary bladder: a clinicopathologic analysis of 29 cases. Am J Surg Pathol 2006;30:883–891.
Lagwinski N, Thomas A, Stephenson AJ, et al. Squamous cell carcinoma of the bladder: a clinicopathologic analysis of 45 cases. Am J Surg Pathol 2007;31:1777–1787.
Ayala AG, Tamboli P, El-Bolkainy MN, et al. Adenocarcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital System, 1st edn. IARC Press: Lyon, France, 2004, pp 128–130.
Halling KC, King W, Sokolova IA, et al. A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J Urol 2000;164:1768–1775.
Jichlinski P . New diagnostic strategies in the detection and staging of bladder cancer. Curr Opin Urol 2003;13:351–355.
Maier U, Simak R, Neuhold N . The clinical value of urinary cytology: 12 years of experience with 615 patients. J Clin Pathol 1995;48:314–317.
Ramakumar S, Bhuiyan J, Besse JA, et al. Comparison of screening methods in the detection of bladder cancer. J Urol 1999;161:388–394.
Saad A, Hanbury DC, McNicholas TA, et al. A study comparing various noninvasive methods of detecting bladder cancer in urine. BJU Int 2002;89:369–373.
Bastacky S, Ibrahim S, Wilczynski SP . The accuracy of urinary cytology in daily practice. Cancer 1999;87:118–128.
Skacel M, Fahmy M, Brainard JA, et al. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J Urol 2003;169:2101–2105.
Halling KC, King W, Sokolova IA, et al. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine. J Urol 2002;167:2001–2006.
Halling KC . Vysis UroVysion for the detection of urothelial carcinoma. Expert Rev Mol Diagn 2003;3:507–519.
Sokolova IA, Halling KC, Jenkins RB, et al. The development of a multitarget, multicolor fluorescence in situ hybridization assay for the detection of urothelial carcinoma in urine. J Mol Diagn 2000;2:116–123.
US Food and Drug Administration Center for Devices and Radiological Health. New Device Approval: Urovysion™ Bladder Cancer: P030052. January 24, 2005.
Daniely M, Rona R, Kaplan T, et al. Combined morphologic and fluorescence in situ hybridization analysis of voided urine samples for the detection and follow-up of bladder cancer in patients with benign urine cytology. Cancer 2007;111:517–524.
Bollmann M, Heller H, Bankfalvi A, et al. Quantitative molecular urinary cytology by fluorescence in situ hybridization: a tool for tailoring surveillance of patients with superficial bladder cancer? BJU Int 2005;95:1219–1225.
Bubendorf L, Grilli B, Sauter G, et al. Multiprobe FISH for enhanced detection of bladder cancer in voided urine specimens and bladder washings. Am J Clin Pathol 2001;116:79–86.
Bergman J, Reznichek RC, Rajfer J . Surveillance of patients with bladder carcinoma using fluorescent in-situ hybridization on bladder washings. BJU Int 2008;101:26–29.
Sugano K, Kakizoe T . Genetic alterations in bladder cancer and their clinical applications in molecular tumor staging. Nat Clin Pract Urol 2006;3:642–652.
Mitra AP, Datar RH, Cote RJ . Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol 2006;24:5552–5564.
Quek ML, Sanderson K, Daneshmand S, et al. New molecular markers for bladder cancer detection. Curr Opin Urol 2004;14:259–264.
Veeramachaneni R, Nordberg ML, Shi R, et al. Evaluation of fluorescence in situ hybridization as an ancillary tool to urine cytology in diagnosing urothelial carcinoma. Diagn Cytopathol 2003;28:301–307.
Yoder BJ, Skacel M, Hedgepeth R, et al. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: a prospective study with focus on the natural history of anticipatory positive findings. Am J Clin Pathol 2007;127:295–301.
Pycha A, Mian C, Posch B, et al. Numerical chromosomal aberrations in muscle invasive squamous cell and transitional cell cancer of the urinary bladder: an alternative to classic prognostic indicators? Urology 1999;53:1005–1010.
Pycha A, Mian C, Posch B, et al. Numerical aberrations of chromosomes 7, 9 and 17 in squamous cell and transitional cell cancer of the bladder: a comparative study performed by fluorescence in situ hybridization. J Urol 1998;160:737–740.
Sawai T, Sasano O, Tsuji T, et al. Chromosome instability evaluated by fluorescence in situ hybridization in hereditary non-polyposis colorectal cancer. J Gastroenterol 1998;33:495–499.
Sawai T, Sasano O, Tsuji T, et al. [Numerical aberration of chromosome 17 is correlated with multiple primary cancer in colorectal carcinoma]. Nippon Shokakibyo Gakkai Zasshi 1997;94:464–468.
Visakorpi T, Hyytinen E, Kallioniemi A, et al. Sensitive detection of chromosome copy number aberrations in prostate cancer by fluorescence in situ hybridization. Am J Pathol 1994;145:624–630.
Das K, Lau W, Sivaswaren C, et al. Chromosomal changes in prostate cancer: a fluorescence in situ hybridization study. Clin Genet 2005;68:40–47.
Varma VA, Fekete PS, Franks MJ, et al. Cytologic features of prostatic adenocarcinoma in urine: a clinicopathologic and immunocytochemical study. Diagn Cytopathol 1988;4:300–305.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reid-Nicholson, M., Ramalingam, P., Adeagbo, B. et al. The use of Urovysion™ fluorescence in situ hybridization in the diagnosis and surveillance of non-urothelial carcinoma of the bladder. Mod Pathol 22, 119–127 (2009). https://doi.org/10.1038/modpathol.2008.179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2008.179