Abstract
This study evaluated the expression patterns of claudins 1, 3, 4, 7, and 8 in human renal cell carcinomas and oncocytomas and correlated expression with patient prognosis. Tissue microarrays were created from paraffin-embedded tissue samples from 141 patients with renal cell carcinomas or oncocytoma (90 clear cell, 22 papillary, 17 chromophobe renal cell carcinomas, and 12 oncocytomas). The staining pattern for claudins 3, 4, 7, and 8 was membranous and/or cytoplasmic, whereas claudin 1 was predominantly membranous in both nonneoplastic renal tissue and tumors. Negative to weak claudin 3 staining was predominantly detected in Fuhrman's grade 1 and 2 clear cell renal cell carcinomas (78%; P=0.016), suggesting that upregulation of claudin 3 potentially occurs concomitantly with increasing grade of clear cell renal cell carcinomas. In addition, Kaplan–Meier univariate analysis showed a significant inverse correlation between moderate to strong claudin 3 and 4 expression with overall survival in clear cell renal cell carcinomas (P=0.038 and P=0.031). Moderate to strong claudin 7 expression was significantly more common in chromophobe renal cell carcinomas (94%) than in oncocytomas (55%; P=0.041). Claudin 8 staining was moderate to strong in 92% of oncocytomas, which differentiated them from papillary and clear cell renal cell carcinomas (14 and 12%; both P<0.0001). Only negative to weak claudin 8 staining was detected in all chromophobe renal cell carcinomas, whereas there were no claudin 8 negative oncocytomas and 8% exhibited a weak staining pattern (P<0.0001). Due to their distinctive expression patterns, claudins 7 and 8 can be used as useful immunohistochemical markers for the separation of chromophobe renal cell carcinomas from oncocytomas, whereas claudins 3 and 4 may serve as indicators of prognosis in clear cell renal cell carcinomas.
Similar content being viewed by others
Main
Renal cell carcinoma consists of approximately 3% of all human malignancies and is the most common renal cancer in adults. Renal cell carcinomas is a heterogeneous group of tumors where histopathological classification, Fuhrman nuclear grade, and tumor stage are commonly used predictors of survival.1, 2, 3 Currently renal neoplasms are classified as follows: clear cell, papillary (chromophil), chromophobe, collecting duct carcinomas, and oncocytoma as well as rarer entities, such as small cell carcinoma, cystic renal cell carcinoma, unclassified and mucinous tubular, and spindle cell carcinoma.2, 3 Clinically, clear cell carcinomas and type II papillary renal cell carcinomas exhibit the highest metastatic potential with lowest survival, whereas type I of papillary renal cell carcinoma as well as chromophobe renal cell carcinomas are usually more indolent in nature.1, 2 However, both papillary and chromophobe renal cell carcinomas may be aggressive and can transform into high-grade tumors with sarcomatoid features.1, 2 On the other hand, oncocytomas exhibit benign clinical behavior, which emphasizes the importance of differentiating them from other renal tumors.4, 5, 6 Namely, the eosinophilic variant of chromophobe renal cell carcinomas as well as granular cell variant of clear cell renal cell carcinomas often morphologically resemble oncocytomas, which renders the diagnosis based on histological features alone difficult. Additional immunohistochemical and/or molecular analyses are thus often warranted for the accurate separation of oncocytomas from other renal tumors, in particular chromophobe renal cell carcinomas.7
Claudins are tight junction proteins that directly affect the magnitude and selectivity of paracellular permeability in epithelial cells, possibly by forming both the barrier and pore of the paracellular pathway.8, 9 More then 20 claudin isoforms have been identified in mammalian species to date and the expression of each appears to be tissue specific.8, 9, 10 Although alterations in the expression of individual claudins have been related to progression and invasion of several human cancers, their role in carcinogenesis remains controversial. Nonetheless, because of the high specificity of claudin expression patterns that has been demonstrated in cancer tissue so far, it was proposed that claudins might represent useful diagnostic and prognostic markers for various cancers.11, 12, 13, 14, 15, 16 Namely, claudins have been identified not only as a useful immunohistochemical markers in distinguishing different variants of neoplasms,11, 12, 13 but their expression was also found to correlate with prognosis in several cancers.14, 15, 16 However, at present only a subset of claudin isoforms have been investigated in a relatively limited number of human cancers. In recent years, a growing number of reports suggest the usefulness of claudin subtype expression in differentiating renal neoplasms.17, 18, 19
In this microarray study, our aim was to investigate the expression pattern of claudins 1, 3, 4, 7, 8 in major subtypes of renal cell carcinomas and oncocytomas, as well as to correlate the expression of these proteins with pathological variables and prognosis in a large series of patients.
Materials and methods
Patients and Specimens
Formalin-fixed paraffin-embedded tissue from 141 patients with renal cell carcinoma or oncocytoma were collected between the years 1999 and 2004 from the archives of the Department of Pathology at the Rhode Island Hospital and the Miriam Hospital. Clinicopathologic features of the patient cohort included in the study are presented in Table 1. Patients included in this study did not receive adjuvant chemotherapy or radiotherapy prior to surgery. Hematoxylin and eosin-stained slides were available and reviewed for adequacy for study and confirmation of diagnosis by two pathologists (ML and LW). Histological diagnoses were confirmed by Hale's colloidal iron stain, and by immunohistochemistry for renal cell carcinomas, CK7, vimentin, α-methylacyl CoA racemase, and c-kit when applicable. Stage of the disease was defined according to American Joint Committee on Cancer criteria. Survival data and progression of disease was accessed through the Rhode Island Tumor Registry and chart review. This study was approved by the Institutional Review Board (IRB nos. 4057–06) at the Rhode Island Hospital.
Tissue Microarray Construction
Tissue microarrays included 90 clear cell renal cell carcinomas, 22 papillary renal cell carcinomas (16 type I and 6 type II), 17 chromophobe renal cell carcinomas (8 conventional and 9 eosinophilic variant), and 12 oncocytomas. The arrays included 4–6 cores (1 mm in diameter) of neoplastic and 2–3 cores of nonneoplastic renal tissue for each case included in this study. Microarrays were constructed using the Manual Tissue Arrayer (Beecher Instruments, Sun Prairie, WI, USA) following the manufacturer's protocol.
Immunohistochemistry
Tissue microarrays 5 mm sections were deparaffinized and rehydrated according to the standard procedures. Microarray sections were subjected to heat and pressure induced antigen retrieval in 10 mM citrate buffer (pH 6.0) for 10 min, blocked using Peroxidase Block (Dako, Carpinteria, CA, USA) for 5 min. Sections were incubated with polyclonal rabbit anti-claudin-1 (1:50), anti-claudin-3 (1:1000); anti-claudin 4 (1:1000); anti-claudin-7 (1;500) antibodies (NeoMarkers, Lab Vision, Fremont, CA, USA) and rabbit anti-claudin-8 (1:100) (GeneTex Inc., San Antonio, TX, USA) overnight at 4°C. After incubation with the appropriate secondary antibodies for 30 min at room temperature antigen–antibody complexes were revealed with standardized development times, using the Envision Plus kit (Dako) for claudins 3, 4, 7, and 8 (Ventana Medical Systems, Tucson, AZ, USA) for claudin 1. To determine the specificity of immunosztaining with the antibodies, control sections were processed without the primary and/or secondary antibodies, which resulted in no labeling. The immunoreactivity was assessed by two pathologists (ML and LW) based on a combined score of the extent and intensity of staining. Scores 0–3 were assigned according to the percentage of positive tumor cells (0=0%; 1=<25%; 2=25–50%; 3=>51%) and the intensity of staining in tumor (0=0; 1=1+; 2=2+; 3=3+). The two scores were multiplied to give an overall score of 0–9, of which 0 was considered negative, 1–2 was considered weak, 3–6 moderate, and 9 strong staining.
Statistical Analysis
Association between the claudins immunohistochemical scores and the histological subtypes of renal cell carcinomas was evaluated by using the χ2-test and/or the Fisher's exact test. For the survival analysis, the staining scores were grouped as negative to weak (overall score 0–2) vs moderate to strong (overall score 3–9) categories. The influence of different clinicopathologic variables on tumor-related survival was assessed using the Kaplan–Meier estimates and subgroups were compared by the logrank (univariate analysis). Multivariate analysis was performed using the Cox's regression model. Two-tailed P-values of 0.05 or less were considered to be statistically significant.
Results
Pattern of Claudin Expression in Nonneoplastic Renal Tissue
Claudin 1 exhibited a predominantly membranous staining pattern (Figure 1a), whereas claudins 3, 4, 7, and 8 demonstrated membranous and cytoplasmic staining patterns in the nonneoplastic renal tissue (Figures 1b–e). In normal renal tissue all of the claudins tested were detected in the distal convoluted tubules and collecting ducts (Figure 1f). Nonetheless several distinctive expression patterns were detected in the claudin staining patterns. Claudin 1 was distinctively detected in Bowman's capsule and the loop of Henle (Figures 1a and f), whereas claudin 3 was expressed strongly in the distal tubules and collecting ducts and weakly in the proximal tubules and glomeruli (Figures 1b and f). In addition to the positive staining in the distal convoluted tubules and collecting ducts, claudin 4 was also expressed weakly in the proximal convoluted tubules. Interestingly, the intensity of claudin 4 staining was comparable to that of claudin 7 and was greater than that of claudin 1 (Figures 1c and f). Claudins 7 and 8 were strongly expressed in the distal convoluted tubules and collecting ducts only (Figures 1d–f).
Pattern of Expression of Claudins 1, 3, and 4 in Neoplastic Renal Tissue
Moderate to strong claudin 1 staining differentiated papillary (64%) from chromphobe renal cell carcinomas (23%; P=0.039), which exhibited predominantly negative to weak staining (Figures 2a and b). Apart from this difference in staining intensity, claudin 1 was found to be evenly expressed in the different renal tumors (Figures 2a and b).
Negative to weak claudin 3 staining was observed in almost all of oncocytomas (92%), which differentiated them from moderate to strong, predominantly membranous or mixed membranous and cytoplasmic staining detected in papillary (82%; P<0.0001), clear cell (38%; P=0.04), and chromophobe renal cell carcinomas (47%; P=0.04) (Figures 3a and b). Moderate to strong claudin 3 staining was also significantly more common in papillary renal cell carcinomas then in clear cell (P=0.0002) and chromophobe renal cell carcinomas (P=0.02) (Figures 3a and b).
Moderate to strong membranous and cytoplasmic claudin 4 expression was present in 88% of the chromophobe tumors and in 55% of the oncocytomas, whereas significantly fewer of the clear cell renal cell carcinomas stained with claudin 4 (50%; P=0.011) (Figures 3a and c). Almost all of the papillary renal cell carcinomas (91%) stained moderate to strongly for claudin 4 (Figures 3a and b).
Pattern of Expression of Claudins 7 and 8 in Neoplastic Renal Tissue
In all types of neoplastic renal tissue, claudin 7 demonstrated a membranous or mixed membranous and diffuse cytoplasmic staining patterns. Interestingly, membranous claudin 7 staining was detected in the majority of chromophobe (78%), clear cell (76%), and papillary renal cell carcinomas (78%), however exclusive membranous staining was present in only 18% of oncocytomas. There was no difference in claudin 7 staining between conventional and eosinophilic variants of chromophobe renal cell carcinoma. Moderate to strong claudin 7 expression was significantly more common in chromophobe renal cell carcinoma (94%) than in oncocytomas (55%) and clear cell renal cell carcinomas (30%) (P=0.041 and P<0.0001, respectively) (Figures 4a–e). On the other hand, 75% of the papillary renal cell carcinomas had moderate to strong positivity for claudin 7, which did not differentiate them from chromophobe renal cell carcinomas. Moreover claudin 7 expression was not significantly higher in the papillary renal cell carcinoma than the expression observed in oncocytomas and clear cell renal cell carcinomas (Figures 4a–e).
Claudin 8 demonstrated moderate to strong diffuse cytoplasmic or mixed cytoplasmic and membranous staining patterns in the vast majority of the oncocytomas (92%), which differentiated them from papillary (14%; P<0.0001) and clear cell renal cell carcinomas (12%; P<0.0001) (Figures 5a–e). An interesting pattern of claudin 8 staining was noted in 27% of oncocytomas where some regions of the tumors demonstrated diffuse cytoplasmic staining, whereas other regions had a typical perinuclear staining pattern not observed in the carcinomas (Figure 5d, inset). Importantly, only negative to weak claudin 8 staining (100%) was detected in chromphobe renal cell carcinomas (Figures 5c and e), and there was no difference in claudin 8 staining between conventional and eosinophilic variants of chromophobe renal cell carcinomas. However, in the oncocytomas there were no negative tumors and only 8% demonstrated weak claudin 8 staining (P<0.0001) (Figures 5d and e).
Expression of claudins 8 in neoplastic renal tissue (original magnification × 200). (a) Clear cell renal cell carcinoma, (b) papillary renal cell carcinoma, (c) chromophobe renal cell carcinoma, (d) oncocytoma. (Inset) Perinuclear staining pattern of claudin 8 in oncocytoma, not observed in carcinomas. (e) Quantification of claudin 8 expression.
Association of Claudin Expression and Nuclear Grading of Renal Neoplasms
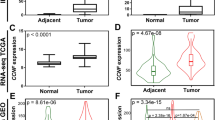
In this study we also investigated whether claudin expression correlated with the nuclear grade of renal cell carcinomas. This analysis showed that negative to weak claudin 3 staining was predominantly detected in Fuhrman's grades 1 and 2 clear cell renal cell carcinomas (78%; P=0.016) (Figure 6). Interestingly, this association was unique for claudin 3 and Fuhrman's grading of clear cell renal cell carcinomas and was not observed in other tested claudins across the different tumor types.
Univariate and Multivariate Survival Analyses
Univariate survival analyses were performed for the association of claudin expression and patient survival across the different types of renal neoplasms. Moderate to strong claudin 3 expression was found to inversely correlate with the overall survival of patients with clear cell renal cell carcinoma (P=0.038, logrank) (Figure 7a). Similarly, moderate to strong claudin 4 expression was also associated with decreased survival in patients with clear cell renal cell carcinoma (P=0.031, logrank) (Figure 7b). Univariate analysis demonstrated also that Fuhrman's grade (P=0.002) and tumor stage (P=0.017) were significant predictors of survival in clear cell renal cell carcinoma (logrank). Multivariate analysis showed the Fuhrman's nuclear grade to be the only independent predictor of survival (P=0.02, Cox test).
In tumor types other than clear cell renal cell carcinoma, claudins 3 and 4 did not correlate significantly with patients outcome (not shown), and expression of other tested claudins did not correlate with patient survival in any of the tumor types.
Discussion
Tight junctions are structures responsible for sealing between the apical portions of adjacent basolateral cell membranes, with an essential role in the control of cellular permeability and maintenance of tissue homeostasis.8, 9 Disruption of the tight junctions is believed to be one of the processes that occur in carcinogenesis allowing for loss of cellular cohesion, aggressive growth, and dedifferentiation of cancer cells.8, 9, 20 Claudins are pivotal proteins in the formation and function of tight junctions, and thus their association with the pathological processes involved in carcinogenesis has been extensively investigated in recent years.20
Our group has previously reported significantly stronger expression of claudin 4 in colonic and gastric neoplastic tissues as opposed to normal mucosa.14, 15 In addition, loss of claudin 1 expression is associated with recurrence and overall decreased survival in stage II colonic cancer.14 Similarly, expression of claudins 3 and 4 was observed in advanced ovarian cancer but not in benign ovarian cystadenomas11 and increased claudins 3 and 4 expression were recently shown to contribute to the aggressive behavior of clear cell and serous papillary endometrial and ovarian serous adenocarcinoma.21, 22 However, a study conducted in patients with surgically treated esophageal carcinomas has found that loss of claudins 3 and 4 expression was associated with the presence of distant metastases,23 indicating again that claudin isoforms indeed may have a highly tissue specific role in carcinogenesis.
In this study we observed that only negative to weak claudin 3 staining was observed in Fuhrman's grades 1 and 2 clear cell renal cell carcinomas, which in some studies has been associated with a more favorable prognosis.24 This finding suggest that upregulation of claudin 3 potentially occurs concomitantly with increasing grade of clear cell carcinomas. In addition, there is a significant inverse correlation between moderate to strong claudins 3 and 4 expression with overall survival in clear cell renal cell carcinomas. This observation opens a possibility for therapeutic targeting of claudins 3 and 4 in patients with higher grades of clear cell carcinomas.20
Oncocytoma, a benign tumor of the kidney, is the most important differential diagnosis of chromophobe renal cell carcinoma; however, the distinction between these two neoplasms may be difficult, especially when considering the eosinophilic variant of chromophobe renal cell carcinoma. Historically, Hale colloidal iron staining and electron microscopy have been the most reliable diagnostic techniques for the separation of chromophobe renal cell carcinoma from oncocytoma.25, 26, 27 However, despite of their diagnostic utility, these techniques are technically demanding and sometimes inconsistent in results or not readily available and costly.
A number of unique immunohistochemical markers are presently being used for differential diagnosis of renal neoplasms.28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 Renal cell carcinoma marker antibody, CD10, glutathione S-transferase-α and others have shown specificity for clear cell renal cell carcinoma,28, 29 α-Methylacyl CoA racemase is expressed in papillary renal cell carcinoma,30 whereas parvalbumin, β-defensin 1 and most recently KIT are used as markers for chromophobe renal cell carcinoma and oncocytoma.31, 32, 33 However a marker with sufficient specificity to differentiate between chromophobe renal cell carcinoma and oncocytomas has not yet been unequivocally established.17 Kidney-specific cadherin was found in one study to be almost exclusively expressed in chromophobe renal cell carcinoma,34 however this finding was not supported by the others who found it also expressed in oncocytomas.35 Similarly, cytokeratin 7 does not appear to show the consistent immunoreactivity in chromophobe renal cell carcinomas as suggested by some, preventing its utility for positive diagnostic differentiation from oncocytomas.36 Inversely, RON proto-oncogene product and S100 protein were found preferentially expressed in oncocytoma warranting further investigation.37, 38
Preclinical studies have demonstrated a developmental expression of claudin isoforms in the renal tissue,39 and have identified a distinct pattern of expression of isoforms 7 and 8 along the mouse distal nephron.10 Gene expression profiling studies in humans have proposed in recent years that chromophobe renal cell carcinoma and oncocytoma are distinguishable by mRNA expression profiles and have indicated claudin 7 as one of the candidate markers for chromophobe renal cell carcinoma.17, 18 Along those lines are also observations of a recent study which indicated that claudin 7 may be a useful immunohistochemical marker in the differential diagnosis of renal neoplasms.19
In our present study we report that in chromophobe renal cell carcinoma 94% of cases showed moderate to strong claudin 7 expression, whereas only negative to weak claudin 8 staining was detected in the same series. Interestingly, there was no difference in claudins 7 and 8 staining between conventional and eosinophilic variants of chromophobe renal cell carcinoma. This is in sharp contrast with renal oncocytomas, in which 45% of the cases were negative to weak for claudin 7 expression, whereas 92% of the cases demonstrated moderate to strong positivity for claudin 8 staining. In addition, no claudin 8 negative cases were detected among the oncocytomas. The combination of claudin 7 positivity and claudin 8 negativity strongly supports a diagnosis of chromophobe renal cell carcinoma. Inversely, claudin 8 positivity and claudin 7 negativity strongly favor oncocytoma. In a subgroup of oncocytomas, but not in the other tumors, we also observed an interesting, perinuclear staining pattern of claudin 8. A similar perinuclear staining pattern was also described earlier in oncocytomas positive for cytokeratin 20 staining,36 and colloidal iron staining (as mentioned above).27 Stopyra et al40 hypothesized that such peculiar staining pattern corresponds to large cytoplasmic condensations of intermediate filaments or globular filamentous bodies, described so far only in pathological processes.
In conclusion, our study has shown distinct expression patterns of claudins 1, 3, 4, 7, and 8 in the renal neoplasms that were consistent with the histogenic origin of the tumors. Our data indicate that claudins 7 and 8 can be used as potentially useful immunohistochemical markers for the separation of chromophobe renal cell carcinomas from oncocytomas. At the same time, claudins 3 and 4 could serve as indicators of prognosis and potential therapeutic targets in patients with clear cell renal cell carcinomas, a notion that warrants further investigation.
References
Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–2540.
Delahunt B, Bethwaite PB, Nacey JN . Outcome prediction for renal cell carcinoma: evaluation of prognostic factors for tumours divided according to histological subtype. Pathology 2007;39:459–465.
Eble JN, Sauter G, Epstein JI, et al. (eds). World Health Organization classification of Tumors. Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs. IARC Press: Lyon, 2004.
Moch H, Gasser T, Amin MB, et al. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma. Cancer 2000;89:604–614.
Cheville JC, Lohse CM, Zincke H, et al. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 2003;27:612–624.
Crotty TB, Farrow GM, Lieber MM . Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol 1995;154:964–967.
Skinnider BF, Amin MB . An immunohistochemical approach to the differential diagnosis of renal tumors. Semin Diagn Pathol 2005;22:51–68.
Angelow S, Yu AS . Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens 2007;16:459–464.
Koval M . Claudins—key pieces in the tight junction puzzle. Cell Commun Adhes 2006;13:127–138.
Li WY, Huey CL, Yu AS . Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol 2004;286:1063–1071.
Rangel LBA, Agarwal R, D'Souza T, et al. Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res 2003;9:2567–2575.
Soini Y, Kinnula V, Kahlos K, et al. Claudins in differential diagnosis between mesothelioma and metastatic adenocarcinoma of the pleura. J Clin Pathol 2006;59:250–254.
Karanjawala ZE, Illei PB, Ashfaq R, et al. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol 2008;32:188–196.
Resnick MB, Konkin T, Routhier J, et al. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol 2005;18:511–518.
Resnick MB, Galivanez M, Newton E, et al. Claudin expression in gastric adenocarcinomas: a tissue microarray study with prognostic correlation. Hum Pathol 2005;36:886–892.
Sheehan GM, Kallakury BV, Sheehan CE, et al. Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas. Hum Pathol 2007;38:564–569.
Rohan S, Tu JJ, Kao J, et al. Gene expression profiling separates chromophobe renal cell carcinoma from oncocytoma and identifies vesicular transport and cell junction proteins as differentially expressed genes. Clin Cancer Res 2006;12:6937–6945.
Hornsby CD, Cohen C, Amin MB, et al. Claudin-7 immunohistochemistry in renal tumors: a candidate marker for chromophobe renal cell carcinoma identified by gene expression profiling. Arch Pathol Lab Med 2007;131:1541–1546.
Li L, Yao JL, di Sant'Agnese PA, et al. Expression of claudin 7 in benign kidney and kidney tumors. Int J Clin Exp Pathol 2008;1:57–64.
Morin PJ . Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res 2005;65:9603–9606.
Choi YL, Kim J, Kwon MJ, et al. Expression profile of tight junction protein claudin 3 and claudin 4 in ovarian serous adenocarcinoma with prognostic correlation. Histol Histopathol 2007;22:1185–1195.
Konecny GE, Agarwal R, Keeney GA, et al. Claudin-3 and claudin-4 expression in serous papillary, clear-cell, and endometrioid endometrial cancer. Gynecol Oncol 2008;109:263–269.
Takala H, Saarnio J, Wiik H, et al. Claudins 1, 3, 4, 5 and 7 in esophageal cancer: loss of claudin 3 and 4 expression is associated with metastatic behavior. APMIS 2007;115:838–847.
Lohse CM, Blute ML, Zincke H, et al. Comparison of standardized and nonstandardized nuclear grade of renal cell carcinoma to predict outcome among 2042 patients. Am J Clin Pathol 2002;118:877–886.
Tickoo SK, Amin MB, Zarbo RJ . Colloidal iron staining in renal epithelial neoplasms, including chromophobe renal cell carcinoma. Am J Surg Pathol 1998;22:419–424.
Murphy WM, Grignon DJ, Perlman EJ . Tumors of the Kidney, Bladder, and Related Urinary Structures. Armed Forces Institute of Pathology: Washington, DC, 2004, pp 101–174. Atlas of Tumor Pathology; Fourth Series, Fascicle 1.
Skinnider BF, Jones EC . Renal oncocytoma and chromophobe renal cell carcinoma. A comparison of colloidal iron staining and electron microscopy. Am J Clin Pathol 1999;111:796–803.
Avery AK, Beckstead J, Renshaw AA, et al. Use of antibodies to RCC and CD10 in the differential diagnosis of renal neoplasm. Am J Surg Pathol 2000;24:203–210.
Chuang ST, Chu P, Sugimura J, et al. Overexpression of glutathione transferase alpha in clear cell renal cell carcinoma. Am J Clin Pathol 2005;123:421–429.
Tretiakova MS, Sahoo S, Takahashi M, et al. Expression of alpha-methylacyl-CoA racemase in papillary renal cell carcinoma. Am J Surg Pathol 2004;28:69–76.
Martignoni G, Pea M, Chilosi M, et al. Parvalbumin is constantly expressed in chromophobe renal carcinoma. Mod Pathol 2001;14:760–767.
Young AN, de Oliveira Salles PG, Lim SD, et al. Beta defensin-1, parvalbumin and vimentin: a panel of diagnostic immunohistochemical markers for renal tumors derived from gene expression profiling studies using cDNA microarrays. Am J Surg Pathol 2003;27:199–205.
Yamazaki K, Sakamoto M, Ohta T, et al. Overexpression of KIT in chromophobe renal cell carcinoma. Oncogene 2003;22:847–852.
Mazal PR, Exner M, Haitel A, et al. Expression of kidney-specific cadherin distinguishes chromophobe renal cell carcinoma from renal oncocytoma. Hum Pathol 2005;36:22–28.
Shen SS, Krishna B, Chirala R, et al. Kidney-specific cadherin, a specific marker for the distal portion of the nephron and related renal neoplasms. Mod Pathol 2005;18:933–940.
Wu SL, Kothari P, Wheeler TM, et al. Cytokeratins 7 and 20 immunoreactivity in chromophobe renal cell carcinomas and renal oncocytomas. Mod Pathol 2002;15:712–717.
Rampino T, Gregorini M, Soccio G, et al. The Ron proto-oncogene product is a phenotypic marker of renal oncocytoma. Am J Surg Pathol 2003;27:779–785.
Lin F, Yang W, Betten M, et al. Expression of S-100 protein in renal cell neoplasms. Hum Pathol 2006;37:462–470.
Abuazza G, Becker A, Williams SS, et al. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol 2006;291:1132–1141.
Stopyra GA, Warhol MJ, Multhaupt HA . Cytokeratin 20 immunoreactivity in renal oncocytomas. J Histochem Cytochem 2001;49:919–920.
Acknowledgements
This study was presented in part at the United States and Canadian Academy of Pathology Annual Meetings in 2007 and 2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lechpammer, M., Resnick, M., Sabo, E. et al. The diagnostic and prognostic utility of claudin expression in renal cell neoplasms. Mod Pathol 21, 1320–1329 (2008). https://doi.org/10.1038/modpathol.2008.116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2008.116
Keywords
This article is cited by
-
Claudin-3 facilitates the progression and mediates the tumorigenic effects of TGF-β in glioblastoma multiforme
Medical Oncology (2023)
-
Downregulation of CLDN7 due to promoter hypermethylation is associated with human clear cell renal cell carcinoma progression and poor prognosis
Journal of Experimental & Clinical Cancer Research (2018)
-
Roles of the first-generation claudin binder, Clostridium perfringens enterotoxin, in the diagnosis and claudin-targeted treatment of epithelium-derived cancers
Pflügers Archiv - European Journal of Physiology (2017)
-
miR-1303 Targets Claudin-18 Gene to Modulate Proliferation and Invasion of Gastric Cancer Cells
Digestive Diseases and Sciences (2014)
-
Claudin expression in high-grade invasive ductal carcinoma of the breast: correlation with the molecular subtype
Modern Pathology (2013)