Abstract
Protective efficacy of Bacillus Calmette-Guérin (BCG) may be affected by the methods and routes of vaccine administration. We have studied the safety and immunogenicity of oral (PO) and/or intradermal (ID) administration of BCG in healthy human subjects. No major safety concerns were detected in the 68 healthy adults vaccinated with PO and/or ID BCG. Although both PO and ID BCG could induce systemic Th1 responses capable of IFN-γ production, ID BCG more strongly induced systemic Th1 responses. In contrast, stronger mucosal responses (TB-specific secretory IgA and bronchoalveolar lavage T cells) were induced by PO BCG vaccination. To generate preliminary data comparing the early gene signatures induced by mucosal and systemic BCG vaccination, CD4+ memory T cells were isolated from subsets of BCG vaccinated subjects pre- (Day 0) and post-vaccination (Days 7 and 56), rested or stimulated with BCG infected dendritic cells, and then studied by Illumina BeadArray transcriptomal analysis. Notably, distinct gene expression profiles were identified both on Day 7 and Day 56 comparing the PO and ID BCG vaccinated groups by GSEA analysis. Future correlation analyses between specific gene expression patterns and distinct mucosal and systemic immune responses induced will be highly informative for TB vaccine development.
Similar content being viewed by others
Introduction
Over one-third of the world’s population is currently infected with Mycobacterium tuberculosis (Mtb), and >1.5 million individuals die of tuberculosis (TB) each year.1 The current TB vaccine, the attenuated Mycobacterium bovis strain Bacillus of Calmette and Guerin (BCG), is effective at preventing disseminated forms of TB in very young children.2, 3 However, the efficacy in prevention of adult pulmonary TB, the major form of transmissible disease, at best is only 50%.4 Intradermal (ID) administration is the major route of BCG vaccination worldwide, but this vaccination method is unlikely to induce optimal lung mucosal immunity.5, 6 We hypothesize that combining systemic and mucosal TB vaccinations may induce not only systemic immunity protective against the worst forms of disseminated TB disease, but also mucosal immunity protective against initial TB infection and/or secondary transmission.
We have initiated a series of BCG vaccination trials with the overall goal of identifying the major human immune responses that BCG does and does not induce, and to determine the immune responses new and improved TB vaccines must stimulate to provide better protection than ID BCG. An additional goal of our ongoing work is to investigate the possibility that mucosal BCG vaccination can uniquely induce immune responses relevant for optimal lung mucosal TB immunity. Because oral (PO) BCG vaccination was the original route used by Calmette and Guerin,3 and millions of persons have been vaccinated with BCG orally in Brazil over the past 50 years,7 there are considerable safety data to support the use of PO BCG in humans.8 In addition, activated memory T and B lymphocytes have been shown to develop differentiation programs allowing them to circulate and home to multiple different mucosal tissues, indicating that PO vaccines could induce increased immunity at distant mucosal sites.9, 10, 11 Administration of ID and/or PO BCG in ongoing research protocols allows for comparison of the mucosal and systemic TB-specific immune responses induced with current and alternative methods of vaccine administration. We report here the first detailed analysis of human immune responses induced by ID and/or PO BCG. Our results support the hypothesis that a combined ID and PO BCG vaccination approach could induce the best combination of mucosal and systemic TB immunity.
Recent studies have used systems biology approaches to obtain a global picture of the immune responses to vaccination in humans.12, 13, 14, 15, 16 Systems biology tools have enabled the identification of early innate signatures that predict the immunogenicity of vaccines, and identification of potentially novel mechanisms of immune regulation. In this work we have taken a novel systems biology approach focusing on the identification of gene expression networks involved in the induction of CD4+ T cells, critically important for optimal mucosal and systemic TB immunity. Our preliminary data indicate that PO and ID BCG induce distinct profiles of molecular signatures, suggesting that more detailed analyses will identify genes and/or gene networks that should be differentially targeted by vaccines designed to induce both optimal mucosal and systemic TB immunity.
Results
Summary of enrollment, safety and determination of optimal PO BCG danish dose
All 16 subjects enrolled into the initial Danish BCG PO dose escalation trial completed 6 months of observation, and none developed any significant adverse events. Optimal BCG-specific blood T cell IFN-γ responses and nasal wash Mtb LAM-specific secretory IgA (sIgA) responses post-vaccination were detected in subjects given 2 × 108 colony forming units (cfu) of Danish BCG PO (data not shown). In the double-blind, placebo controlled trial, 60 subjects were randomized among the groups A-F (10/group; see Figure 1 and Supplementary Figure 1 online). In addition, 8 subjects were enrolled into an open label arm (group G) who received 2 × 106 cfu of Connaught BCG ID. All 68 subjects received their initial BCG vaccinations as planned, 63 subjects were followed for at least 6 months after the initial vaccinations, 43 received second vaccinations 1 year later (20 received BCG revaccination and 23 received placebo 1 year after the first vaccinations), and 48 completed 2 years of follow-up. The number of drop outs during the second year reduced sample sizes to less than optimal for detection of significant increases in the immunogenicity parameters induced by the booster BCG vaccinations. Therefore, for most immunogenicity results presented below, groups A+B, C+D and E+F were combined based on the type of BCG vaccination given at the beginning of the trial. Only limited comparisons between group G and the other groups are shown because of the smaller sample size in group G, further reduction in sample size due to drop outs, and the lack of meaningful differences detected between groups A+B (given ID Danish BCG) and group G. However, some group G subjects were recruited into a BAL study and these results are presented along with the results from all other groups. No deaths or other significant adverse events (SAE) occurred during these trials. There were no severe AEs caused by vaccination except for in 1 subject (ID BCG alone/Group A) who developed transient erythema at the ID vaccination site graded as severe by size. This severe graded erythema did not interfere with the subject’s normal activities and was not associated with any other symptoms. There were eleven moderate related unsolicited adverse events, nine of which occurred post vaccination 1. Seven of these events, experienced by subjects in all Groups except PO BCG alone (groups C+D), were described as continued reactogenicity. The remaining four events, experienced by subjects in ID BCG alone, PO BCG alone and combined ID and PO groups (1 subject in each of the following groups: A, B, C and E), included: appetite loss, upper respiratory infection, URI and mandibular pain. Overall, these results indicate that no safety signals were detected in this small trial.
Overview schematic of the clinical trial. Ten subjects were recruited into each of the groups (A–F), randomized in a double blind fashion to receive PO vs. ID vs. PO+ID Danish BCG. An additional 8 subjects were recruited into an open label, ID Connaught BCG vaccination cohort (to compare ID immunity induced by Danish vs. Connaught BCG). During year 1 of the trial, groups A and B were identical (given only ID BCG), groups C and D were identical (given only PO BCG), and groups E and F were identical (given both ID and PO BCG). Blood, tear, nasal wash and stool samples were collected at 9 different time points (pre-vaccination, and 1 week, 2 months, 6 months, 12 months, 12 months plus 1 week, 14 months, 18 months and 24 months post-vaccination). *10 volunteers/group
Mtb-specific lymphoproliferative responses were induced most strongly by ID BCG
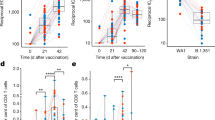
Supplementary Figure 2A. presents the mean±s.e. peripheral blood lymphoproliferative responses induced in all subjects regardless of group assignment over the 2 years of follow-up. BCG significantly increased (P<0.05) Mtb-specific T-cell responses that persisted for at least 2 years after vaccination. Lymphoproliferative responses specific for a single major Mtb secreted protein (Ag85B), and for all Mtb secreted proteins present in culture filtrates (CF) of logarithmically replicating extracellular Mtb organisms in vitro, were both significantly increased post-vaccination. Figure 2 shows lymphoproliferative responses induced by these same Mtb antigen preparations (Ag85B and Mtb CF in Figure 2a and b, respectively) during the first year post-vaccination for subjects given ID BCG alone (groups A+B), PO BCG alone (groups C+D) and both ID and PO BCG (groups E+F). Median responses for each are shown at the different time points. These latter results demonstrate that all three BCG vaccination strategies resulted in at least some increased Mtb-specific lymphoproliferative responses. However, only subjects given ID BCG (with or without PO BCG) developed significantly higher post-vaccination responses.
Blood lymphocyte Mtb-specific lymphoproliferation assay results. Heparinized whole-blood samples were diluted with medium 10-fold and stimulated with either the major secreted Ag85 protein (a) or Mtb culture filtrate (CF, complex lysate of all Mtb antigens secreted by exponentially growing organisms), (b), and tritiated thymidine incorporation measured on day 7. Median values for each group (N=20) are shown for each time point. *P<0.05, **P<0.01 by Wilcoxon-matched pairs testing compared with pre-vaccination responses.
Mtb-specific IFN-γ-producing T cells were most strongly induced by ID BCG
Supplementary Figure 2B online. presents the mean±s.e. peripheral blood IFN-γ ELISPOT responses induced, as an overall assessment of Mtb-specific type 1 immune responses, in all subjects regardless of group assignment over the 2 years of follow up. BCG vaccination also significantly increased Mtb-specific type 1 T cells that persisted for at least 2 years after vaccination. IFN-γ responses specific for the major secreted protein, Ag85B, and for all Mtb CF secreted proteins, were both significantly increased post-vaccination. Figure 3 shows IFN-γ ELISPOT responses induced by Ag85B and Mtb CF (Figure 3a and b, respectively) during the first year post-vaccination for subjects given ID BCG alone (groups A+B), PO BCG alone (groups C+D) and both ID and PO BCG (groups E+F). Median responses are depicted for each group at the different time points. It can be seen that only the groups given ID BCG (with or without PO BCG) developed significantly increased Mtb-specific IFN-γ responses post-vaccination (by Wilcoxon matched pairs test). Table 1 further confirms these results demonstrating that significantly increased proportions of subjects in the groups given ID BCG developed positive IFN-γ responses post-vaccination (defined as a response greater than the mean plus 2 SE of all baseline responses; all significant differences detected are presented). In 5 different comparisons of antigen-specific responses at both early and late time points, ID groups had significantly higher proportions of responders than PO recipients. Furthermore, 2 comparisons indicated that combined ID plus PO BCG vaccination induced higher proportions of positive IFN-γ responses than PO BCG alone, providing additional support for the conclusion that ID BCG induces stronger systemic TB-specific immunity than PO BCG. Two comparisons indicated that ID BCG alone induced higher proportions of responders than ID plus PO, perhaps because more T cells were recruited out of the blood and into the peripheral mucosal tissues, due to the higher doses of BCG given PO (40-fold more BCG cfu were used for PO compared with ID vaccination).
Blood lymphocyte Mtb-specific IFN-γ ELISPOT assay results. Freshly harvested PBMC were studied in IFN-γ ELISPOT plates stimulated with an Ag85 overlapping peptide pool (a) or Mtb CF (b). Median values for each group (N=20) are shown for each time point. *P<0.05, **P<0.01 by Wilcoxon matched pairs testing compared with pre-vaccination responses.
Mucosal Mtb-specific sIgA responses were most strongly induced by PO BCG
We studied Mtb-derived lipoarabinomannan (LAM)-specific sIgA in stool, nasal wash and tear samples pre- and post-vaccination, as a marker of BCG-induced mucosal immunity. As reported previously,17 we were unable to measure vaccine-induced, LAM-specific sIgA responses in stool samples, presumably due to dilutional and proteolytic effects known to occur as sIgA is transported to the distal gut. However, in nasal wash samples (Figure 4a) and tears (Figure 4b.), relative increases in Mtb LAM-specific sIgA were seen post-vaccination in the groups that had received PO BCG, but not in the group given only ID BCG. These relative increases compared to Day 0 seen in the PO BCG vaccination groups did not achieve statistical significance (by Wilcoxon matched pairs tests), probably because of the small sample sizes. However, our previous data generated from 2 PO BCG dose escalation trials demonstrated that PO BCG can induce TB-specific sIgA responses.9, 17 These results indicate that PO BCG can uniquely induce mucosal immune responses relevant for TB immunity.
Mtb-specific secretory IgA results. Nasal wash (a) and tear (b) samples were obtained and studied in Mtb lipoarabinomannan (LAM)-coated ELISA plates. In both cases, subjects given PO BCG were found to have higher Mtb LAM-specific sIgA detected. Each group (N=20).
Mtb-specific T cells were increased in the lungs of subjects given PO BCG
As a sub-study to the main BCG vaccination protocol, we performed BAL on 16 consenting subjects (5 received ID Danish BCG alone, 3 received ID Connaught BCG alone, 6 received PO Danish BCG alone, and 2 received both ID and PO Danish BCG), 1–12 months after the initial vaccinations. BAL cells were collected and studied by both IFN-γ ELISPOT and flow cytometric methods. We stimulated the BAL cells with Ag85B peptides, purified protein derivative (PPD) and live BCG as previously described.18 The results from two subjects were excluded from analysis because the low viability of recovered BAL cells resulted in no response even to the positive controls, and the few negative values obtained were replaced with ‘0’. Figure 5a shows that similar circulating blood lymphocyte IFN-γ responses were detected comparing all subjects given ID BCG alone and all subjects given PO BCG with or without ID BCG. Despite similar circulating blood lymphocyte responses, Figure 5b demonstrates that up to sixfold more Mtb-specific T cells were found in the airways of subjects who received PO BCG compared with subjects given only ID BCG. Figure 5a,b present separate symbols for individual subjects given ID Danish BCG only (blue circles), ID Connaught BCG only (purple triangles), PO Danish BCG only (orange circles) and ID plus PO Danish BCG (green squares). Trends for increased immunogenicity of ID Connaught BCG compared with ID Danish BCG, and increased immunogenicity of ID plus PO Danish BCG compared with PO Danish BCG alone, can be observed both with blood lymphocytes and BAL cells. However, these trends (additional variables) do not alter our overall conclusions that ID BCG induced similar or stronger blood responses than PO BCG, while PO BCG induced stronger BAL responses. Furthermore, both ID BCG alone and PO±ID BCG, induced significant increases in Mtb-specific blood responses compared with the matched medium rest background responses for the same group, while only PO±ID BCG induced significant increases in Mtb-specific BAL responses compared with medium rest (P<0.05 by Wilcoxon matched pairs testing).
Bronchoalveolar lavage (BAL) sub-study to identify lung TB-specific T cells. A sub-study targeting subjects enrolled in the parent DMID-01-351 trial was conducted involving a single post-vaccination BAL procedure and blood sample obtained the same day. (a,b) show IFN-γ ELISPOT results for PBMC and BAL cells, respectively. Box-plots with scatter plot overlay are presented for PBMC and BAL cells. (c) shows flow cytometric studies identifying the percentages of CD4+ and CD8+ T cells expressing α4β1 in PBMC. (d) Flow cytometric studies identifying the percentages of CD4+ and CD8+ T cells expressing CXCR3 among BAL cells. *P<0.05, **P<0.01 by Mann–Whitney testing compared with group responses.
We also studied T-cell surface expression of α4β1 and CXCR3, known to be involved in immune trafficking to the lung. In Figure 5c., we show that the proportions of circulating blood CD8+ T cells expressing the integrin complex α4β1 were significantly increased in subjects vaccinated with PO BCG. In Figure 5d., we demonstrate that significantly increased proportions of lung CD4+ and CD8+ T cells in subjects given PO BCG expressed CXCR3, a chemokine receptor previously implicated in lung trafficking of antigen-specific T cells.19, 20 These results indicate that PO BCG can increase the numbers of circulating blood T cells that express α4β1 suggesting that assessment of circulating α4β1+ T cells may be useful as a biomarker of the induction of mucosal responses. In addition, the expression of CXCR3 on lung airway T cells may be useful as a biomarker of recent lung trafficking.
Cutaneous PPD-specific DTH responses were induced only by ID BCG
We previously reported that PO BCG skewed Mtb-specific T cells toward mucosal homing and reduced cutaneous homing manifest as significantly reduced PPD-specific delayed-type hypersensitivity (DTH) reactivity in PO compared with ID BCG recipients.21 At the end of the 2 years of observation, we performed 5 TU PPD skin tests and found, as reported before, that there was a significantly greater proportion of subjects given ID BCG with positive PPD responses (⩾10 mm) compared with subjects given PO BCG (9/18 vs. 0/13, respectively, P<0.05 by Fisher’s exact two-sided test). Only 3 of 13 given ID and PO BCG had positive PPD responses at the end of the trial, also consistent with negative effects of PO BCG on PPD-specific DTH. These results confirm our previous studies indicating that ID and PO BCG program vaccine-induced T cells for differential cutaneous vs. mucosal homing programs.22
ID and PO BCG-induced distinct memory CD4+ T cell transcriptomal responses
Because we found unique mucosal and systemic immune responses induced by PO and ID BCG, respectively, we performed additional studies of the CD4+ T-cell transcriptomes present post-vaccination. We hypothesized that distinct individual genes and gene networks important for differential mucosal and systemic immune responses would be induced by the different routes of vaccination. We compared vaccine-induced transcriptomal changes at the predicted peak of post-vaccination T-cell activation (Day 7 post-vaccination), and at an early memory time point (Day 56 post-vaccination). We focused on comparisons of transcriptomes at Day 7 directly ex vivo without further in vitro stimulation to restrict our studies to the ongoing responses induced in vivo. However, at Day 56 we assumed that memory T cells would be past the peak of in vivo activation and require further stimulation in vitro to identify differences induced by PO and ID BCG vaccination. Figure 6 shows the results of our preliminary transcriptomal investigations. Figure 6a shows individual heat maps for the top 100 most significantly altered genes comparing Day 0 and Day 7 responses directly ex vivo (without re-stimulation with BCG-infected dendritic cells), in CD4+ T cells from the PO and ID BCG vaccinated subjects. The Venn diagram in Figure 6b demonstrates that the Day 7 differentially expressed genes were largely distinct comparing subjects given PO and ID BCG vaccination. Figure 6c shows individual heat maps for the top 100 most differentially expressed genes comparing Day 0 and Day 56 responses after in vitro re-stimulation with BCG-infected autologous dendritic cells, in CD4+ T cells from PO and ID BCG vaccinated subjects. The Venn diagram in Figure 6d demonstrates that similar to Day 7 responses, the Day 56 differentially expressed genes were largely distinct comparing subjects given PO and ID BCG vaccination. These results provide strong evidence that PO and ID BCG induce very different transcriptomal patterns in human CD4+ T cells important for differential induction and persistence of mucosal and systemic TB immunity.
CD4+ T-cell transcriptomal differences induced by PO and ID BCG. Memory CD4+ T cells were purified from PBMC collected pre-vaccination, 7 days post-vaccination and 56 days post-vaccination from 11 subjects (4 given PO BCG and 7 given ID BCG). Autologous dendritic cells (DC) were generated from blood monocytes and infected or not with BCG. The three different serial memory CD4+ T-cell populations were cultured for 24 h with uninfected or BCG-infected DC, and then RNA collected for Illumina BeadArray transcriptomal analysis. (a) Heat maps for the top 100 altered genes comparing Day 7 and Day 0 direct ex vivo responses (PO N=4 and ID N=7). (b) This panel shows that only three of the top altered genes detected at Day 7 were the same in PO vs. ID BCG recipients. (c) Heat maps for the top altered genes comparing Day 56 and Day 0 after re-stimulation with BCG-infected DC (PO N=4 and ID N=7). (d) This panel shows that only 2 of the top altered genes detected at Day 56 were the same in PO vs. ID BCG recipients.
Discussion
Our major original hypothesis was that the combination of mucosal and systemic BCG vaccination would induce the optimal combination of mucosal and systemic TB-specific immune responses important for TB protective immunity. Overall the results of this trial support this conclusion by indicating that PO BCG-induced stronger mucosal responses and ID BCG-induced stronger systemic immune responses. Furthermore, the combination of PO and ID BCG resulted increases in both mucosal and systemic immune responses. In addition, no major safety signals were identified, further supporting the rationale for additional studies of mucosal BCG vaccination.
ID BCG vaccination induced stronger and more reproducible systemic immune responses compared with PO BCG vaccination. Positive response rates post-vaccination in both PBMC IFN-γ ELISPOT and lymphoproliferative assays, were greater in subjects given ID compared with PO BCG. Numerous examples of ID recipients developing significantly higher response rates than PO recipients were seen in these assays that measure functional characteristics of systemic CD4+ T helper type 1 responses thought to be critical for TB protective immunity. In Figure 3a, groups E+F are shown to have increased responses at Day 7, but then the responses are decreased. The increased responses at Day 7 in subjects given both PO+ID BCG suggest early induction of T cells, but these induced T cells may traffic to mucosal peripheral sites leading to decreased numbers of T cells circulating in the blood.
PO BCG vaccination induced stronger/more reproducible mucosal secretory IgA responses compared with ID BCG vaccination. Positive response rates in the LAM-specific secretory IgA responses detected in nasal wash and tear specimens collected post-vaccination were higher in subjects given PO BCG compared with ID BCG alone. These relative increases compared to Day 0 seen in the PO BCG vaccination groups did not achieve statistical significance (by Wilcoxon matched pairs tests), probably because of the small sample sizes. However, combined with our previously published data from PO dose escalation trials reporting that PO BCG can significantly increase TB-specific sIgA responses,9, 17 our current results further support the unique induction of nasal wash and tear sIgA responses by PO BCG. In contrast, despite these results, the stool secretory IgA responses were not increased in PO BCG recipients. Initially, this might seem surprising because the PO BCG delivery is expected to induce gut mucosal immunity after being taken up by mucosal immune inductive sites within the Peyer’s patches lining the small bowel. However, our current negative results for stool secretory IgA after PO BCG are similar to the findings in our 2 previous PO BCG trials, in which we have not detected post-vaccination increases in stool secretory IgA responses. Stool contains a high content of proteases which can degrade secretory IgA over time,23 and the method for collection of stool samples results in much higher dilutions of stool secretions compared with the methods for collection of nasal washes and tears.17 Therefore, stool studies are the least reliable for detection of mucosal immune responses induced by vaccination compared with other mucosal secretions. The detection of increased secretory IgA responses in nasal washes and tears document mucosal immune responses induced by PO BCG, and also demonstrate that these mucosal immune responses were able to traffic to distant mucosal tissues including those lining the upper respiratory track, possibly relevant for protection against TB infection.
The comparison of BAL cell and PBMC responses in subjects recruited for the BAL sub-study demonstrated that PO BCG also induced highly significant increases in T-cell surface markers important for lymphocyte trafficking to the lung. Highly reproducible and significant increases in both BAL T cell expression of CXCR3 and PBMC T cell expression of the α4/β1 integrin complex were induced in subjects given PO with or without ID BCG, compared with those given ID BCG alone. These T cell surface markers have been shown in numerous model systems to be important for the trafficking of CD4+ Th1 cells and CD8+ T cells to the lungs.6, 11, 20, 24, 25 Both CD4+ Th1 and CD8+ T cells specific for TB antigens are thought to be critically important for protective TB immunity.6 Therefore, the BAL/PBMC sub-study results indicate that PO BCG induces the potential for more T cells protective against TB to traffic to the site of initial infection.
Also, evident in the BAL/PBMC sub-study, PO BCG with or without ID BCG led to higher BAL overall TB-specific T cell responses in the lungs compared with persons given only ID BCG. The numbers of antigen-specific BAL T cells producing IFN-γ after in vitro stimulation with Ag85, PPD and BCG were four- to sixfold increased in persons given PO with or without ID BCG. The fact that reproducible increases were detected with all TB antigens used to stimulate T cell responses in vitro, further supports our conclusion. Despite the reproducible increases in TB-specific T cells in subjects given PO BCG, the responses in PBMC obtained from these subjects on the same day as the BAL procedures were similar in subjects given ID BCG alone and subjects given PO BCG with or without ID BCG. These latter results provide additional support for the conclusion that PO BCG uniquely induced increased TB-specific T cells with lung-trafficking potential. The BAL procedures were done 1–12 months post-vaccination in these subjects, further indicating the robustness of our findings. These results, combined with the sIgA and lymphocyte trafficking molecule expression results discussed above, provide complementary evidence that PO BCG more strongly induces multiple mucosal immune responses relevant for protective mucosal TB immunity.
We have hypothesized previously21 that PO BCG induces differential T-cell trafficking compared with ID BCG. The above findings are consistent with this hypothesis. In addition, the PPD-specific DTH responses detected in subjects at the end of this trial support our earlier published results,21 indicating that PO BCG fails to induce PPD-specific DTH responses, and even may suppress these responses. These results support our earlier conclusion that PO BCG could provide TB immunity against initial infection without the induction of PPD-specific delayed type hypersensitivity that can interfere with detection of latent TB infection and disease.
The preliminary CD4+ T-cell transcriptomal studies produced provocative data suggesting that PO and ID BCG vaccination induce distinct profiles of molecular signatures, which may explain the differences in mucosal and systemic immune responses induced. The sample size studied in this manner needs to be expanded with additional stored PBMC to further confirm the validity of our conclusions, and to allow for studies of correlations between the expression of specific genes and gene sets and the mucosal and systemic immune responses induced in this trial. This work is currently ongoing.
Further research in this area should focus on at least two major areas to learn how optimal TB-specific mucosal and systemic immunity can be induced. First, we have only explored one combined schedule of PO and ID BCG vaccination. Additional work could investigate the effects of different doses of PO and ID BCG, different strains of BCG given PO and ID, different sequences of mucosal vs. systemic BCG vaccination (e.g., first priming with mucosal vaccination followed at different intervals by ID vaccination), different routes of mucosal BCG vaccination (intranasal or aerosolized vaccinations with BCG may induce even stronger TB-specific lung immunity, although additional safety concerns would need to be addressed), and mucosal and systemic vaccinations with newer and potentially more potent TB vaccines. Second, additional studies investigating the molecular events which drive the induction of optimal mucosal and systemic TB-specific immune responses, are needed to allow further iterative improvements in mucosal and systemic vaccinations. To this end, the preliminary CD4+ T-cell transcriptomal studies in subjects given PO vs. ID BCG vaccination need to be expanded as noted above.
Overall, these results demonstrate that ID BCG induces the strongest TB-specific systemic immune responses, while PO BCG induces the strongest TB-specific mucosal immune responses. Therefore, these results support our original hypothesis that mucosal and systemic vaccinations with BCG will induce the optimal combination of mucosal and systemic immune responses relevant for protection against TB infection and disease progression. In addition, these results will greatly facilitate further targeted development of methods for assessing mucosal and systemic immune responses induced by other TB vaccines.
Methods
Subject enrollment, randomization and follow up. Healthy HIV-negative, HCV-negative, Quantiferon TB-negative adults aged 18–45 years without known TB exposure risks or immunosuppression were enrolled into 2 separate trials. The first (DMID-99-024) involved a bridging dose escalation trial of Danish 1331 BCG given orally to 16 subjects at 1 of 3 doses: 2 × 108 cfu, 2 × 109 cfu or 2 × 1010 cfu. The second trial (DMID-01-351) involved 68 subjects randomized into a double-blind, placebo-controlled comparison of Danish or Connaught BCG given intradermally (ID), orally (PO) or by both routes. Subjects were randomized to Groups A-F (10/group) to receive Danish SSI BCG or placebo at Day 0 and 1 year later. To explore the BCG strain specificity of clinical effects and immunogenicity eight volunteers were enrolled into an open label group given ID Connaught BCG. Each ID BCG dose included 5 × 105 cfu, and each PO BCG dose included 2 × 108 cfu. ID BCG was delivered over the deltoid in 0.1 ml of saline. PO BCG was delivered in 60 ml of PBS within 15 min after the subject ingested 150 ml of oral bicarbonate (0.1 M) solution given to neutralize stomach acid. After drinking the BCG, subjects immediately ingested an additional 150 ml of PBS alone to ensure all BCG was swallowed. Solicited symptoms were collected for 15 days (Days 0–14) after each vaccination. Unsolicited adverse events were collected for 2 months after each vaccination. Serious adverse events were collected through 12 months after the last vaccination. Immediately before vaccination and at various times after each vaccination (1 week, 2 months, 6 months and 12 months), samples of serum, nasal washes, tears and stool were obtained for the assessment of IgG and IgA responses by ELISA. In addition, PBMC were collected at these same time points pre- and post-vaccination for analyses of lymphoproliferative, cytokine ELISPOT and transcriptomal assays. As a sub-study, bronchoscopy and bronchoalveolar lavage (BAL) were performed on a subset of subjects at 1–12 months following the primary and/or secondary vaccination.
Lymphoproliferation. Whole blood samples were diluted 10-fold with RPMI and expanded with optimal doses of recombinant Ag85b protein, Mtb culture filtrate which was derived from the H37Rv strain and contains most of the secreted proteins from the organism (BEI Resources), PPD, live BCG, or rested in medium for 7 days at 37 °C in 5% CO2. During the last 10–14 hours, these cultures were pulsed with tritiated thymidine and cell-incorporated radioactivity measured. The results are presented as disintegrations per minute (d.p.m.) of radioactivity.
IFN-γ ELISPOT. IFN-γ producing cells were identified by ELISPOT using ImmunoSpot plates (Cellular Technology, Cleveland, OH, USA) and IFN-γ-specific antibodies (BD Pharmingen, San Diego, CA, USA). PBMC or total bronchoalveolar lavage cells (0.2–1 × 105 cells per well) were stimulated with Ag85b peptide pools (1 μg ml−1), recombinant Ag85b protein (5 μg ml−1), Mtb culture filtrate (5 μg ml−1, BEI) live BCG (0.5 MOI) or medium alone for 24 h at 37 °C with 5% CO2. Spots were identified by CTL Analyzer and ImmunoSpot software, version 3.2 (C.T.L.).
Secretory IgA (sIgA) ELISA. Nasal washes, tears and stool samples were collected as described previously.17 Immulon 2 plates were coated with 4 μg ml−1 of lipoarabinomannan H37Ra LAM (provided by Dr. Larry Schlesinger, Ohio State University) diluted in 100% ethanol. The plates were dried for 3–4 h in a fume hood then washed with PBS plus 0.05% tween 20. Plates were blocked overnight at 4 oC with 1% BSA in PBS with 0.05% tween 20. Following blocking, the plates were washed and serum, tear, nasal wash and stool samples were added to duplicate wells at optimal dilutions predetermined for each type of sample. The plates were incubated overnight at 4 oC. After washing and the application of goat anti-human IgG or goat anti-human IgA horseradish peroxidase (Southern Biotechnology Associates), plates were incubated for 1 h at room temperature in the dark. Then plates were washed and the ABTS substrate (Kirkegaard & Perry, Gaithersburg, MD), was added. After the plates were incubated 20 min at room temperature, absorbance was read at 405 nm.
Bronchoalveolar lavage. A sub-study was conducted to analyze T cells in the lungs of subjects vaccinated with BCG ID and/or PO. Subjects were recruited for a single bronchoscopy with BAL 1–12 months post-vaccination. A blood sample for density-gradient purification of PBMC was obtained on the day of the BAL procedure in order to compare T cell responses in the lung and blood. The BAL cells were centrifuged at 485 × g and washed with medium. 2–300 000 PBMC/BAL cells were placed in individual wells of IFN-γ ELISPOT plates and studied as described above. Additional aliquots of PBMC and BAL cells were stained for surface expression of CD3, CD4, CD8, CXCR3 and α4β1, analyzed by flow cytometry and Flow Jo software.
Transcriptomal analyses. To perform a preliminary study of the molecular signatures of CD4+ T cells induced by ID vs. PO BCG vaccination, we purified CD4+ T cells with Miltenyi immunomagnetic beads and prepared dendritic cells (DC) for use as antigen presenting cells as described previously.26 We purified CD4+ T cells from PBMC harvested before vaccination (naïve control samples), 7 days after vaccination (representing the peak of T cell activation after BCG in vivo) and 56 days after vaccination (representing an early memory time point of BCG immunity). Autologous DC were infected or not with Danish BCG (MOI of 20) and then co-cultured overnight with CD4+ T cells purified from pre- and post-vaccination PBMC. RNA was extracted the next day with RNAeasy Mini Kit (Qiagen, Valencia, CA, USA). Therefore, 6 RNA samples were studied for each volunteer individually. Illumina Bead Arrays were used to generate CD4 transcriptomes within the high throughput Microarray Core at Washington University. Data were normalized and studied with GenePattern and GSEA to compare pre- and post-vaccination transcriptomes directly ex vivo and after BCG in vitro re-stimulation.27, 28 The differential analysis was done by ComparativeMarkerSelection v10, and two-sided T tests with 10 000 permutations were used to identify gene expressions.
PPD-specific DTH responses. To measure PPD-specific DTH responses 2 years after BCG vaccination, 5 TU of Tubersol was applied intradermally and mm of induration measured 48–72 h later.
Statistical analyses. Statistical analyses were performed by the EMMES Corporation. The Wilcoxon matched pairs non-parametric test was used to compare pre- to post-vaccination responses. Fisher’s exact tests were used to compare proportions of positive responses and the Mann–Whitney U test was used to compare responses between groups. Statistical analyses were performed using SAS version 9.3 software (2011 SAS Institute Inc., Cary, NC).
References
WHO Global tuberculosis report 2013. Available from http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1 (2013) Accessed 7 January 2015.
Colditz, G.A. et al. Efficacy of BCG vaccine in the prevention of Tuberculosis: Meta-analysis of the published literature. JAMA 271, 698–702 (1994).
Calmette, A., Weill-Halle, B., Saenz, A. & Costil, L. Demonstration experimentale du passage des bacilles-vaccines BCG a travers la muquesuse del'intestin chez l'enfant et chez le singe. Bull. Acad. Med. 110, 203–212 (1933).
Trunz, B.B., Fine, P. & Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367, 1173–1180 (2006).
Andersen, P. & Doherty, T.M. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 3, 656–662 (2005).
Feng, C.G. & Britton, W.J. CD4+ and CD8+ T cells mediate adoptive immunity to aerosol infection of Mycobacterium bovis bacillus Calmette-Guerin. J Infect Dis. 181, 1846–1849 (2000).
de Assis, A. Vaccination concomitante au BCG. 1st Internat. Cong. BCG. Annales de l'Institut Pasteur/Actualites 75, 205–225 (1948).
Monteiro-Maia, R. & Pinho, R.T. Oral bacillus Calmette-Guerin vaccine against tuberculosis: why not? Mem. Inst. Oswaldo Cruz. 109, 838–845 (2014).
Gheorghiu, M., Lagranderie, M., Balazuc, A.M. & Gicquel, B. Mucosal induced immune responses after oral BCG vaccination. Clinical Immunology and Immunopathology 76, 547 (1995).
Belyakov, I.M. et al. Antigen-specific B-cell responses in mucosal immunity after oral administration of BCG. FASEB 9th International Congress of Immunology #4780 (1995) (Unpublished).
Ancelet, L.R., Aldwell, F.E., Rich, F.J. & Kirman, J.R. Oral vaccination with lipid-formulated BCG induces a long-lived, multifunctional CD4(+) T cell memory immune response. PLoS ONE 7, e45888 (2012).
Petrovsky, N., Schonbach, C. & Brusic, V. Bioinformatic strategies for better understanding of immune function. In silico Biol. 3, 411–416 (2003).
Querec, T.D. et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 10, 116–125 (2009).
Pulendran, B., Li, S. & Nakaya, H.I. Systems vaccinology. Immunity 33, 516–529 (2010).
Nakaya, H.I. et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 12, 786–795 (2011).
Trautmann, L. & Sekaly, R.P. Solving vaccine mysteries: a systems biology perspective. Nat. Immunol. 12, 729–731 (2011).
Brown, R.M. et al. Lipoarabinomannan-reactive human secretory immunoglobulin A responses induced by mucosal bacille Calmette-Guerin vaccination. J. Infect. Dis. 187, 513–517 (2003).
Silver, R.F. et al. Recruitment of antigen-specific Th1-like responses to the human lung following bronchoscopic segmental challenge with purified protein derivative of Mycobacterium tuberculosis. Am. J. Respir. Cell Mol. Biol. 29, 117–123 (2003).
Walrath, J., Zukowski, L., Krywiak, A. & Silver, RF. Resident Th1-like effector memory cells in pulmonary recall responses to Mycobacterium tuberculosis. Am. J. Respir. Cell Mol. Biol. 33, 48–55 (2005).
Walrath, J.R., Lee, H. & Silver, R.F. The role of α4β1 (VLA4) in recruitment of Mycobacterium tuberculosis -specific recall responses to the human lung. Am. J. Resp. Crit. Care Med. 175, A101 (2007).
Hoft, D.F., Brown, R. & Belshe, R.B. Mucosal BCG vaccination in humans inhibits delayed-type hypersensitivity to PPD, but induces mycobacterial specific IFN-γ responses. Clin. Infect. Dis. 30, S217–S222 (2000).
Hoft, D.F. & Tennant, J.M. Persistence and boosting of bacille Calmette-Guerin-induced delayed-type hypersensitivity. Ann. Intern. Med. 131, 32–36 (1999).
Ferguson, A., Humphreys, K.A. & Croft, N.M. Technical report: results of immunological tests on faecal extracts are likely to be extremely misleading. Clin. Exp. Immunol. 99, 70–75 (1995).
Agostini, C. et al. CXC chemokines IP-10 and mig expression and direct migration of pulmonary CD8+/CXCR3+ T cells in the lungs of patients with HIV infection and T-cell alveolitis. Am. J. Resp. Crit. Care Med. 162 (4 Pt 1), 1466–1473 (2000).
Du, G. et al. TCR repertoire, clonal dominance, and pulmonary trafficking of mycobacterium-specific CD4+ and CD8+ T effector cells in immunity against tuberculosis. J Immunol. 185, 3940–3947 (2010).
Hoft, D.F. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet 372, 164–175 (2008).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102, 15545–15550 (2005).
Reich, M., Liefeld, T., Gould, J., Lerner, J., Tamayo, P. & Mesirov, JP. GenePattern 2.0. Nat. Genet. 38, 500–501 (2006).
Acknowledgements
We thank the following DMID investigators for their help with protocol design and development (Dr Christine Sizemore, Dr Mamodikoe Makhene, and Ms Robin Mason). This project has been funded by the National Institutes of Health with funding from R01HL111523 and VTEU award HHSN272200800003C.
Author contributions
D.F.H., M.X., A.B., and J.T. contributed to the implementation of the study and supervision at the study site. G.L.Z. and V.B. contributed to transcriptomal data analysis; all other statistical analyses were performed by T.J.D. and H.H. from the EMMES Corporation. D.F.H. was the principal investigator. P.L.A., V.B. contributed to the study design. C.K. and G.M. performed bronchoscopy and bronchoalveolar lavage on vaccinated subjects. L.S.S. provided LAM for sIgA ELISA, contributed to the data interpretation and the writing and approval of the report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Hoft, D., Xia, M., Zhang, G. et al. PO and ID BCG vaccination in humans induce distinct mucosal and systemic immune responses and CD4+ T cell transcriptomal molecular signatures. Mucosal Immunol 11, 486–495 (2018). https://doi.org/10.1038/mi.2017.67
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2017.67
This article is cited by
-
Safety and immunogenicity of a thermostable ID93 + GLA-SE tuberculosis vaccine candidate in healthy adults
Nature Communications (2023)
-
Distinct gene expression signatures comparing latent tuberculosis infection with different routes of Bacillus Calmette-Guérin vaccination
Nature Communications (2023)
-
Characterization of local and circulating bovine γδ T cell responses to respiratory BCG vaccination
Scientific Reports (2019)
-
Dissecting the heterogeneity of DENV vaccine-elicited cellular immunity using single-cell RNA sequencing and metabolic profiling
Nature Communications (2019)
-
Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques
Nature Medicine (2019)