Abstract
Intranasal inoculation with influenza hemagglutinin subunit with polyinosine-polycytidylic (polyI:C), a synthetic analog for double-stranded RNA, enhances production of vaccine-specific immunoglobulin (Ig) A, which is superior to IgG in prophylactic immunity. The mechanism whereby polyI:C skews to IgA production in the nasal-associated lymph tissue (NALT) was investigated in mouse models. Nasally instilled polyI:C was endocytosed into CD103+ dendritic cells (DCs) and induced T-cell activation, including interferon (IFN)-γ production. According to knockout mouse studies, polyI:C activated the Toll-like receptor 3 signal via the adapter TICAM-1 (also called TRIF), that mainly caused T-cell-dependent IgA production. Nasal CD103+ DCs activated transforming growth factor-β signaling and activation-induced cytidine deaminase upon polyI:C stimulation. IgA rather than IgG production was impaired in Batf3−/− mice, where CD103+ DCs are defective. Genomic recombination occurred in IgA-producing cells in association with polyI:C-stimulated DCs and nasal microenvironment. PolyI:C induced B-cell-activating factor expression and weakly triggered T-cell-independent IgA production. PolyI:C simultaneously activated mitochondrial antiviral signaling and then type I IFN receptor pathways, which only minimally participated in IgA production. Taken together, CD103+ DCs in NALT are indispensable for the adjuvant activity of polyI:C in enhancing vaccine-specific IgA induction and protective immunity against influenza viruses.
Similar content being viewed by others
Introduction
Influenza is an uncontrolled epidemic infectious disease caused by influenza virus that occurs worldwide every year. The inactivated split-vaccine against influenza virus has been administered subcutaneously to elicit anti-hemagglutinin (HA)-specific immunoglobulin (Ig) G in serum, while the cold-adapted live influenza vaccine has been administered via intranasal (i.n.) inoculation to induce anti-HA-specific secretory immunoglobulin A (sIgA) in the nasal mucosa and upper respiratory tract.1 Although antigen (Ag)-specific IgG induced by vaccination is effective against homologous virus, it poorly crossreacts with heterogeneous strains. In contrast, sIgA induced by i.n. vaccination efficiently crossreacts with off-target strains.1 Thus sIgA induction is important in eliciting high levels of protection against different strains of influenza virus. Strategies to produce sIgA with adjuvant would be required for prophylactic vaccination against influenza.
Polyinosine-polycytidylic (polyI:C) and polyI:polyC12U, synthetic analogs of double-stranded RNA, act as adjuvants to effectively induce Ag-specific sIgA production in the nasal cavity when they are administrated via i.n. inoculation with a split-product HA vaccine.2, 3 PolyI:C is recognized by endosomal Toll-like receptor 3 (TLR3) and cytosolic retinoic acid-inducible gene-I-like receptors, including melanoma differentiation-associated protein 5 and retinoic acid-inducible gene-I, to signal via Toll/IL-1β receptor homology domain-containing adaptor molecule (TICAM)-1 and mitochondrial antiviral signaling protein (MAVS), respectively.4, 5 The activation of these signaling pathways induces production of type I interferon (IFN) and inflammatory cytokines, leading to the activation of host immunity, including Ig production.6 Nasal vaccination of HA would provoke IgA class switch in B cells through unknown events involving innate adjuvanticity of polyI:C.
IgA class switching recombination (CSR) is initiated by the expression of α germline transcripts and requires activation-induced cytidine deaminase (AID) and categorized into T-cell-dependent (TD) and independent (TI) pathways.7 The TD pathway requires cytokine (transforming growth factor (TGF)-β, interleukin (IL)-4, IL-5 and IL-6) signaling and binding of CD40 on B cells to its ligand CD40L on T cells.7, 8 The TI pathway is activated by B-cell-activating factor (BAFF), a proliferation-inducing ligand (APRIL),9 and TLR ligands instead of CD40 signaling. BAFF, APRIL, and the TLR4 ligand lipopolysaccaride induce nuclear factor (NF)-κB activation to express AID,7, 10 which is an initiation protein for Ig class switching in B cells.11
Mucosal dendritic cells (DCs) have a pivotal role for the induction of TI and TD IgA production. In the gastrointestinal tract, tumor necrosis factor-α and inducible nitric oxide synthase double-positive DCs and plasmacytoid DCs highly express BAFF and APRIL and regulate IgA production.12, 13 TLR5-expressing CD103+CD11b+ DCs sense commensal bacteria and express retinaldehyde dehydrogenase 2, resulting in the induction of IgA+ plasma cells.14 In lung tissue, retinaldehyde dehydrogenase 2-expressing CD103+CD11b− and CD103−CD11b+CD24+ DCs induce IgA CSR in a retinoic acid and TGF-β-dependent manner.15 Compared with gut-associated lymph tissue and lung tissue, IgA-producing mechanisms and DC subsets involved in IgA induction have been poorly investigated in oral and nasal mucosa. The nasal-associated lymphoid tissue (NALT), located in the i.n. cavity, is an inductive site for mucosal immunity in the upper respiratory tract. Multiple immune cells, including T cells, B cells, macrophages, and DCs, exist in the NALT and elicit immune responses against not only invading microbes but also vaccines.16, 17, 18 Although i.n. inoculation of polyI:C induces the expression of cytokines in the respiratory tract,19 it is unclear what immune cells are activated in NALT in response to i.n. inoculation of polyI:C to elicit effective vaccination.
In this study, we aimed at elucidating how polyI:C enhances vaccine-specific IgA production in the nasal cavity and the role of CD103+ DCs, T cells and B cells in IgA CSR in NALT.
Results
PolyI:C enhances vaccine-specific IgA production in NALT through the TICAM-1 pathway
We first tested whether i.n. inoculation of polyI:C promoted IgA production by vaccination. Wild-type (WT) C57B6/J mice were immunized via i.n. inoculation with A/California/07/2009 (H1N1) split-vaccine with or without polyI:C. Two weeks after the second vaccination, we harvested nasal wash (NW) and serum from the mice. The levels of anti-HA IgA in NW and anti-HA IgG in serum were measured by enzyme-linked immunosorbent assay (ELISA). Although vaccine treatment alone did not induce IgA in NW, vaccine treatment with polyI:C significantly enhanced IgA production (Figure 1a). IgG production in serum was modestly induced by vaccine treatment alone and significantly enhanced by polyI:C (Figure 1b). Thus polyI:C mostly governs IgA production in the nasal mucosa and largely affects IgG production. We examined the proportion of IgA+ B cells in lymph node (LN) after vaccination (Figure 1c,d and Supplementary Figure S1a online). Inoculation of polyI:C barely brought increases of B220+ cells to the spleen, LNs, and NALT (Figure 1c and Supplementary Figure S1b). However, the proportion of IgA+ B220+ cells was increased specifically in NALT after polyI:C inoculation (Figure 1d). IgA+ B cells actually accumulated in NALT prepared from vaccinated WT mice after vaccination with polyI:C (see Supplementary Figure S1c). The proportion of IgA+ B220+ cells in the spleen, mesenteric LN, and cervical LN was unchanged by polyI:C treatment (Figure 1c,d). Total numbers of B220+ B cells were similar in NALT irrespective of polyI:C inoculation (see Supplementary Figure S1b). PolyI:C-enhanced Ig production was abrogated in CD4+ T-depleted mice (Figure 1e), suggesting that CD4+ T-cell help is crucial for the enhancement of Ig production by polyI:C.
Polyinosine-polycytidylic (polyI:C) enhances vaccine-specific Ig production. C57B6/J mice (n=5) were vaccinated with 1 μg of split-hemagglutinin (HA) vaccine in the presence or absence of 3 μg of polyI:C. Four weeks later, mice were re-immunized by the same recipe. Two weeks after the second immunization, nasal wash (NW) fluid and serum were collected to measure HA-specific immunoglobulin A (IgA) (a) and IgG (b) production by enzyme-linked immunosorbent assay. **P<0.01, in Mann–Whitney U test. (c, d) Tissues were collected from vaccinated mice and cells were isolated by mechanical disruption. Cells were stained with fluorescein isothiocyanate–labeled anti-IgA, phycoerythrin-labeled anti-B220, and allophycocyanin-labeled anti-CD45 antibodies (Abs) and subjected to flow cytometric analysis. The percentages of B220+ cells in CD45+ cells are shown in c. The percentages of IgA+ cells in B220+CD45+ cells are shown in (d). CLN, cervical lymph node; MLN, mesenteric lymph node; NALT, nasal-associated lymph node. **P<0.01, in Student’s t-test. (e) Mice were pretreated with anti-CD4 (GK1.5) Ab on day before vaccination. After second vaccination, NW fluid and serum were collected to measure vaccine-specific IgA and IgG production. **P<0.01; *P<0.05 in Mann–Whitney U-test. The bars show average of 4–5 mice for each group.
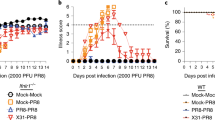
Next, to clarify the signaling pathway responsible for the adjuvanticity of polyI:C, we immunized vaccine to Ticam1−/− and Mavs−/− mice with or without i.n. inoculation of polyI:C. Ticam1−/− mice abolished polyI:C-dependent IgA production in comparison with WT or Mavs−/− mice (Figure 2a,b). In parallel, the numbers of IgA+ B cells in NALT from Ticam1−/− mice vaccinated with polyI:C was decreased in contrast to those in WT mice (see Supplementary Figure S1c). The total IgA+ B cells and B220+ B cells were unchanged by Ticam1−/− in mice (see Supplementary Figure S1c,d). Similar but less prominent tendencies were observed in IgG production in Ticam1−/− mice (Figure 2b). The polyI:C-augmented production of IgA and IgG was impaired in Tlr3−/− mice (Figure 2c). In contrast, Mavs disruption did not affect the production of IgA and IgG (Figure 2a,b). Although the type I IFN induction largely covers adjuvanticity of polyI:C as reported,6, 20 Ifnar−/− mice, where the type I IFN receptor (IFNAR) is defective, produced almost the same amounts of vaccine-specific IgA in NW and IgG in serum as per WT mice (Figure 2d). Interferon regulatory factor 3 (IRF3) is the transcription factor mainly involved in TLR3-mediated type I IFN production,21 but Irf3−/− mice showed no defect in either IgA or IgG production by polyI:C (Figure 2d). Thus the i.n. inoculation of polyI:C strongly augments the production of vaccine-specific IgA and IgG through the TLR3-TICAM-1 pathway independent of MAVS, IRF3, and type I IFN. Actually, polyI:C and HA vaccine exhibited sufficient prophylactic effect on H1NI influenza virus infection in WT mice, whereas the same recipe exhibited far less effect in Ticam1−/− mice, judged by body weight and survival (Figure 2e,f), the results being consistent with those previously reported.2
The Toll-like receptor 3–Toll/IL-1β receptor homology domain-containing adaptor molecule 1 (TICAM-1) pathway is indispensable for polyinosine-polycytidylic (polyI:C)-enhanced vaccine-specific immunoglobulin A (IgA) production. (a–d) As shown in Figure 1, wild-type (WT) and indicated gene-deficient mice (n=3–10) were intranasally (i.n.) vaccinated twice and production levels of vaccine-specific IgA in nasal wash (NW) and IgG in serum were measured by enzyme-linked immunosorbent assay. The bars show average of each group. *P<0.05; **P<0.01; ***P<0.001 and NS, not significant in Mann–Whitney U-test. (e, f) WT or Ticam1−/− mice (n=5) were i.n. vaccinated twice with hemagglutinin vaccine alone or with polyI:C inoculation on days 0 and 28. Fourteen days after inoculation, mice were challenged with 250 plaque-forming units of PR8 strain. (e) Body weight changes and (f) survival rates were monitored every day for 14 days, and mice were killed when they reached the ethical end point of 30% loss of their initial body weight. Data represent one of two independent experiments with similar results. *P<0.05 in Logrank test for trend in WT vaccine and polyI:C vs. Tic KO vaccine and polyI:C. PBS, phosphate-buffered saline.
Ag-specific T-cell response is induced through the TICAM-1 pathway
To examine whether polyI:C potentiates Ag-specific T-cell responses, we prepared splenocytes from the immunized mice and restimulated the cells with the split-vaccine (Figure 3). With polyI:C, vaccine significantly enhanced IFN-γ production, which is an indicator of Ag-specific T-cell responses (Figure 3a–c). The levels of IFN-γ and the ratios of IFN-γ+CD4+ T and CD8+ T cells in splenocytes were incremented in response to Ag restimulation and comparable between polyI:C-treated Mavs−/− and WT mice (Figure 3d–f). In contrast, the increases of these parameters were abrogated in splenocytes prepared from polyI:C-treated Ticam1−/− mice (Figure 3d–f). Tlr3−/− mice also impaired the induction of Ag-specific IFN-γ production in splenic T cells (data not shown). Although the levels of Ig production were indistinguishable in Ifnar−/−, Irf3−/−, and WT mice (Figure 2d), Ag-specific T-cell response was abrogated in Ifnar−/− and Irf3−/− mice (Figure 3g,h). Taken together, polyI:C augments Ag-specific T-cell responses through the TLR3-TICAM-1 pathway. Type I IFN and IRF3 are dispensable for Ig production but optionally required for T-cell response in nasal vaccine in support of the polyI:C adjuvanticity.
Antigen-specific T-cell response depended on the Toll-like receptor 3–Toll/IL-1β receptor homology domain-containing adaptor molecule 1 pathway. Wild-type (WT) and the indicated gene-deficient mice were intranasally vaccinated twice and splenocytes were re-stimulated by 0–10 μg ml−1 of split-hemagglutinin vaccine for 3 days. (a, d, and g) The levels of interferon (IFN)-γ production in culture supernatants were determined by enzyme-linked immunosorbent assay. Cells were incubated with brefeldin A during the last 6 h of culture and stained with phycoerythrin (PE)-Cy7-labeled anti-CD3, fluorescein isothiocyanate–labeled anti-CD4, PE-labeled anti-CD8, and allophycocyanin-labeled anti-IFN-γ antibodies. (b, c, e, f, and h) The percentages of IFN-γ-producing CD3+CD4+ T cells and CD3+CD8+ T cells are shown. The values are presented as the mean±s.d. of three samples for each group. *P<0.05; **P<0.01; ***P<0.001 and NS, not significant in Student’s t-test (a–c), one-way analysis of variance test with Bonferroni’s multiple comparison post hoc test (d–h). PBS, phosphate-buffered saline; PolyI:C, polyinosine-polycytidylic.
CD103+DCs in NALT express TLR3 and are activated by polyI:C
To clarify the mechanisms by which polyI:C enhances IgA production in the nasal cavity, we tried to identify the TLR3-expressing cells in NALT. TLR3 is restrictedly expressed in certain DC subsets, such as CD8α+ and CD103+ DCs in mouse.22, 23 Actually, we found that TLR3 expression in CD11b+ or CD11c+ cells were selectively diminished in NALT of the Tlr3−/− mice (see Supplementary Figure S2a). As no CD8α+ DCs were detected in NALT (see Supplementary Figure S2b), we focused on CD103+ DCs for further analysis. The results from flow cytometric and immunohistochemical analysis using an anti-TLR3 antibody (Ab) showed that CD11b−CD11c+CD103+ DCs (CD103+ DCs) expressed TLR3 in NALT, as well as other CD11c+ cells (Figure 4a,b). The TLR3 expression was confirmed by reverse transcriptase–PCR in CD103+ DCs in NALT (Figure 4c).
CD103+ dendritic cells (DCs) express Toll-like receptor 3 (TLR3) and respond to polyinosine-polycytidylic stimulation. (a) Gating scheme for analysis of macrophage and DC subset in nasal-associated lymph node (NALT). Single-cell suspension isolated from NALT was analyzed for the expression of the indicated makers. TLR3 expression gated on the CD11c−CD11b+ or CD11c+CD11b−CD103+ regions were examined. Class-matched isotype control antibody (Ab) was used as negative control. One representative experiment out of three is shown. (b) NALT sections were stained with allophycocyanin (APC)-labeled anti-TLR3 and fluorescein isothiocyanate (FITC)-labeled anti-CD11c or FITC-labeled anti-CD103 Abs and then and analyzed by fluorescent microscopy. Scale bar shows 10 μm. Class-matched isotype control Ab was used as a negative control. One representative experiment out of two is shown. (c) Expression of TLRs on CD11c+CD103+ and CD11c+CD103− DCs isolated form NALT. NALT DCs and mesenteric lymph node (MLN) DCs were isolated using cell sorter. The Tlr3 mRNA level was determined by reverse transcriptase–PCR. First PCR products of NALT DCs were used as templates for second PCR. MLN CD8α+ DCs were used as positive control. One representative experiment out of two is shown. (d, e) NALT sections were prepared from mice inoculated with 0.5 μg of rhodamine-polyI:C (R-PIC) for 2 h and stained with FITC-labeled anti-CD11c or FITC-labeled CD103 and APC-labeled anti-TLR3 Abs. Stained sections were monitored by fluorescent microscopy. Scale bar shows 5 μm. Class-matched isotype control Ab was used as negative control. One representative experiment out of two is shown. (f) Confocal microscopy of R-PIC-loaded CD103+CD11c+ cells stained for TLR3 (green) and 4,6-diamidino-2-phenylindole (DAPI; blue). CD103+CD11c+ DCs were collected altogether from the spleen, NALT, mesenteric, cervical, and axillary and inguinal LNs as described in Methods section. Isolated cells were co-cultured with R-PIC on ice for 30 min, washed with cold phosphate-buffered saline (PBS) twice and then incubated 37 °C for 30 min. Cells were fixed and stained with FITC-labeled anti-TLR3 Ab. Scale bar shows 10 μm. One representative experiment out of two is shown. FSC, forward scatter; SSC, side scatter.
We next posed the question about whether polyI:C is incorporated into TLR3-expressing DCs. Mice were administered with rhodamine-polyI:C (R-PIC) via i.n. inoculation. After 2 h, NALT sections were obtained and stained with anti-CD11c and anti-TLR3 Abs. R-PIC was incorporated into CD11c+ DCs and colocalized with endosomal TLR3 (Figure 4d,e). CD103+ cells also took up R-PIC, which was colocalized with endosomal TLR3 (Figure 4d,e). Isolated CD103+ DCs were able to incorporate polyI:C into TLR3 endosome in vitro (Figure 4f). Thus at least TLR3-positive CD103+ DCs participate in polyI:C response.
To investigate whether nasal inoculation with polyI:C activates CD103+ DCs in NALT, we assessed the activation of DCs after i.n. polyI:C inoculation. CD86 and major histocompatibility complex class II, activation makers of DCs, were upregulated on CD11c+ and CD103+ DCs in NALT in response to polyI:C (Figure 5a). Although CD11c+ DC activation occurred in Ticam1−/− mice, the activation of CD103+ DCs was abolished in Ticam1−/− but not in Mavs−/− mice (Figure 5b). These findings by fluorescence-activated cell sorting analysis further endorse the imaging results that CD103+ DCs in NALT take up polyI:C to mature through the TLR3-TICAM-1 pathway. The activation levels of Ticam1−/− CD11c+ and CD103+ DCs stimulated by cholera toxin (CT) were comparable to those of WT DCs (data not shown), suggesting that Ticam1−/− CD11c+ and CD103+ DCs equivalently respond to an adjuvant that does not require TICAM-1.
The activation of dendritic cells (DCs) and B cells in nasal-associated lymph node (NALT) after vaccination. (a) Flow cytometry of wild-type NALT DC subpopulations stained for allophycocyanin-labeled anti-CD45, phycoerythrin (PE)-Cy7-labeled anti-CD11c, PE-labeled anti-CD103, and fluorescein isothiocyanate (FITC)-labeled atni-CD86 or FITC-labeled anti-major histocompatibility complex (anti-MHC) class II antibodies at 24 h after vaccination. Upper and lower panels show DC subpopulation gated on CD45+CD11c+ and CD45+CD11c+CD103+ cells, respectively. (b) Flow cytometric analysis of NALT DC subpopulations prepared from vaccinated Mavs−/− and Ticam1−/− mice. One representative experiment out of three is shown. PolyI:C, polyinosine-polycytidylic.
CD103+ DCs are indispensable for polyI:C-enhanced IgA production
Basic leucine zipper transcription factor ATF-like 3 (BATF3) is an essential transcriptional factor in the development of CD8α+ and CD103+ DCs.24 Batf3−/− mice exhibit loss of CD8α+ and CD103+ DCs in LNs.24 To examine the involvement of CD103+ DCs in Ig production, we evaluated IgA and IgG induction in Batf3−/− mice. CD103+ cells in NALT were abolished in Batf3−/− mice (Figure 6a). IgA induction by polyI:C was largely and IgG production in serum was less significantly decreased in Batf3−/− mice than in WT mice (Figure 6b). Thus BATF3-dependent pathway is important for enhancement of IgA and, to a lesser extent, IgG production by polyI:C.
Immunoglobulin A (IgA) and IgG production reduces in Batf3−/− mice. (a) Flow cytometric analysis of mesenteric lymph node (MLN), inguinal lymph nodes (ILN), and nasal-associated lymph node (NALT) dendritic cell (DC) subset gated on 7AAD− CD45+CD11c+ cells. Gated regions indicate CD11c+CD103+ DCs. (b) Wild-type (WT) and Batf3−/− mice (n=3–10/group) were intranasally vaccinated twice and the production levels of vaccine-specific IgA in nasal wash (NW) and IgG in serum were measured by enzyme-linked immunosorbent assay. The values are presented as the mean±s.d. of 3–10 mice for each group. (c) At 2 weeks after the second vaccination, splenocytes were re-stimulated with split hemagglutinin vaccine (0–10 μg ml−1) for 3 days and the proportion of interferon (IFN)-γ+ cells in CD4+ or CD8+ T cells was analyzed by flow cytometer. The values are presented as the mean±s.d. of three mice for each group. **P<0.01; ***P<0.001 and NS, not significant in one-way analysis of variance test with Bonferroni’s multiple comparison post hoc test. PolyI:C, polyinosine-polycytidylic; SSC, side scatter.
The numbers and ratios of IgA+ B cells in polyI:C-treated Batf3−/− NALT were also decreased compared with those in WT (see Supplementary Figure S1a,c), whereas the total numbers of B220+ B cells were unchanged after polyI:C treatment (see Supplementary Figure S1d). Furthermore, the polyI:C-enhanced T-cell response was abolished in Batf3−/− mice (Figure 6c). Thus CD103+ DCs in NALT are essential for the upregulation of Ag-specific IgA production and Ag-specific T-cell responses in response to i.n. inoculation of polyI:C. As Batf3−/− mice lack CD8α+ and CD103+ DCs, which are involved in cross-presentation, they may impair Ag-specific CD8+ T-cell response.24 In addition, Batf3−/− mice also failed to induce Ag-specific CD4+ T-cell response in this study (Figure 6c). CD103+ DCs therefore support the function of CD8+ T as well as CD4+ T cells in nasal vaccination.
As B cells marginally express TLR3 in NALT (Figure 7a), we next examined whether polyI:C directly targets B cells for activation. PolyI:C and vaccine inoculation induced subtle activation of B cells in NALT prepared from WT and Mavs−/− mice but less induced in NALT from Ticam1−/− mice (Figure 7b,c). However, B-cell activation was comparable in between Batf3−/− and WT mice (Figure 7b,c). Therefore, minor B-cell activation occurs independent of CD103+ DCs but depending on TICAM-1, suggesting that i.n. inoculation of polyI:C participates in a direct route for B-cell activation in NALT, which appears through the TLR3 pathway. However, the direct activation of B cells via the TLR3 pathway by polyI:C far less contributes to IgA production than CD103+ DCs in response to polyI:C. In vitro IgA production in Ticam1−/− and Batf3−/− B cells was normal relative to WT B cells (Figure 7d,e). Furthermore, CT, a representative mucosal adjuvant independent of the TLR3-TICAM-1 pathway, similarly induced IgA production and IgA+ B cells in NALT in WT, Tlr3−/−, and Batf3−/− mice (see Supplementary Figure S3). These data suggest that there is no global defect in Ab-producing ability in Ticam1−/− mice and the B cells normally respond to CT for IgA production in Ticam1−/− and Batf3−/− mice.
B-cell activation in nasal-associated lymph node (NALT) by polyinosine-polycytidylic (polyI:C). (a) Toll-like receptor 3 (TLR3) expression in NALT B cells was detected by flow cytometry using anti-TLR3 antibody (Ab). Class-matched isotype control Ab was used as control. One representative experiment out of two is shown. (b) Flow cytometric analysis of B-cell activation in NALT. The percentage of CD69+ cells to CD45+B220+ cells was measured by flow cytometry of wild-type (WT) or knockout (KO) NALT B cells stained for allophycocyanin-labeled anti-CD45, phycoerythrin-labeled anti-B220, and fluorescein isothiocyanate–labeled anti-CD69 Abs at 24 h after vaccination. One representative experiment out of three is shown. (c) The graph shows the average percentage of CD69+ B cells in the indicated knockout mice. The values are presented as the mean±s.d. of three mice for each group. *P<0.05 and NS, not significant in one-way analysis of variance with Bonferroni’s multiple comparison post hoc test. (d, e) B cells from WT, Ticam1−/−, or Batf3−/− were co-cultured with anti-CD40 and anti-immunoglobulin M (anti-IgM) in the presence or absence of mesenteric lymph node (MLN) dendritic cells (DCs) for 5 days and IgA+ B cells were quantified. (d) Representative flow cytometric plots. (e) The graph shows the percentage of IgA+ B cells in the indicated KO mice (n=4). *P<0.05 and NS, not significant in Mann–Whitney U-test. The indicated numbers show the percentages of the gated population.
Germinal center (GC) formation in NALT was enhanced by polyI:C
Next we examined the effect of polyI:C on GC formation in NALT. CD4+PD-1+CXCR5+ and B220+GL7+Fas+ are reported as markers for representing follicular T (Tfh) and GC B cells, respectively.25 PolyI:C significantly increased both proportions and numbers of GC B and Tfh cells in WT mice after vaccination with polyI:C (Figure 8a–f). Treatment with PolyI:C failed to promote the GC formation in Ticam1−/− mice (Figure 8a–f), whereas polyI:C weakly increased the proportion of Tfh cells in Batf3−/− mice after vaccination with polyI:C (Figure 8d,e). The numbers of Tfh cells were not significantly upregulated after polyI:C treatment in Batf3−/− mice (Figure 8f). Hence, the NALT IgA response occurs by vaccination with vaccine and polyI:C in a T-cell-mediated context in GC under the DC network, which is abrogated in NALT of Ticam1−/− or Batf3−/− mice. Ticam1−/− mice have normal T-cell responses after influenza infection,26 suggesting that T-cell responses in Ticam1−/− mice are sufficient in infection or Ticam1-independent stimuli, but selectively abrogated in polyI:C stimulation, that acts mainly on DCs.
Polyinosine-polycytidylic (PolyI:C) enhances the formation of germinal center via Toll/IL-1β receptor homology domain-containing adaptor molecule 1 (TICAM-1). (a) Flow cytometric profiles of nasal-associated lymph node (NALT) germinal center (GC) B cells. GC B cells were stained with allophycocyanin (APC)-labeled anti-CD45, phycoerythrin (PE)-Cy7-labeled anti-Fas, PerCPCy5.5-labeled anti-GL7, and fluorescein isothiocyanate (FITC)-labeled anti-B220 antibodies (Abs) at 2 weeks after vaccination. One representative experiment out of four is shown. (b) The percentage of GC B cells. (c) The number of GC B cells. (b) Flow cytometry of NALT follicular T (Tfh) cells stained for APC-labeled anti-PD-1, PE-Cy7-labeled anti-CD3, PerCPCy5.5-labeled anti-CXCR5, Alexa700-labeled anti-CD45, and FITC-labeled anti-CD4 Abs at 2 weeks after vaccination. One representative experiment out of four is shown. (e) The percentage of Tfh cells. (f) The number of Tfh cells. The values are presented as the mean±s.d. of four mice for each group. *P<0.05; **P<0.01 and NS, not significant in Student’s t-test. The indicated numbers show the percentages of gated population.
TLR3-TICAM-1 pathway induces IgA class switch genes in NALT
As TGF-β signaling in B cells and cytokines are crucial for the induction of TD IgA CSR,7, 8 we measured the expression of TGF-β-related genes and cytokines in NALT after vaccination. PolyI:C did not induce Tgfb1, 2, and 3 mRNA expression in NALT (Figure 9a). Metalloprotease 9 (Mmp9), a protease that converts inactive TGF-β to the active form to engage TGF-β receptors,27 was induced by vaccine and polyI:C inoculation (Figure 9b). Serpine peptidase inhibitor, clade E, member 1 (Serpine1) and TGF-β-induced protein (Tgfbi), which are TGF-β-inducible genes,28, 29 were also upregulated by vaccine and polyI:C inoculation (Figure 9b). The expression of Mmp9, Serpine1, and Tgfbi mRNA in response to vaccine and polyI:C stimulation was abrogated in Tlr3−/− mice (Figure 9b). To confirm activation of TGF-β signaling in B cells, we examined phosphorylation of Smad2 protein in NALT by flow cytometric analysis. The phosphorylated Smad2 protein was detected in B220+ B cells in WT mice by treatment with vaccine and polyI:C, while phosphorylated Smad2 protein was barely detected in Ticam1−/− mice (Figure 9c). Moreover, the expression of cytokines involved in IgA class switching in NALT was upregulated in WT but not in Ticam1−/− (data not shown) or Tlr3−/− mice in response to polyI:C (Figure 9d). IL-21, which is one of the effector cytokines for formation of GC,30 was upregulated in WT but not in Tlr3−/− mice upon polyI:C inoculation (Figure 9d). PolyI:C-induced Ifn-β mRNA expression in Ticam1−/− (data not shown) or Tlr3−/− mice was comparable to that in WT mice (Figure 9d), suggesting participation of the MAVS pathway. The expression of CD40L on T cells and CD40 on B cells remained unchanged after vaccination with polyI:C (Figure 9e).
Gene expression involving immunoglobulin class switching recombination. (a) The expression of transforming growth factor (TGF)-β, (b) TGF-β-related genes, and (d) cytokines in nasal-associated lymph node (NALT) was measured by quantitative PCR at 6 h after intranasal (i.n.) inoculation of 1 μg vaccine with or without 5 μg polyinosine-polycytidylic (polyI:C). Data are mean±s.d. of three mice for each group. *P<0.05; **P<0.01 in Student’s t-test between vaccine alone and vaccine with polyI:C in respective mouse strains. (c) Flow cytometry of NALT B cells stained with phycoerythrin-labeled anti-phosphorylated Smad2 (pSmad2). B cells were gated with fluorescein isothiocyanate–labeled anti-B220 and allophycocyanin-labeled anti-CD45 antibodies (Abs). Panel shows pSmad2 expression level in CD45+B220+ cells in NALT at 6 h after i.n. inoculation of vaccine with or without polyI:C. One representative experiment out of three is shown. (e) The expression of CD40 on B cells and CD40L on T cells in NALT at 2 weeks after second vaccination. One representative experiment out of three is shown. (f) Flow cytometric analysis of NALT CD45+CD11c+CD103− dendritic cells (DCs) and CD45+CD11c+CD103+ DCs stained with anti-B-cell-activating factor (anti-BAFF) or anti-a proliferation-inducing ligand (anti-APRIL) Abs. One representative experiment out of three is shown. (g) Flow cytometric analysis for expression of BAFF and APRIL in wild-type (WT) or Tlr3−/− NALT DCs at 24 h after i.n. vaccination. The graph shows the average percentage of BAFF+ or APRIL+ in CD103+ DCs cells in the indicated knockout mice. The values are presented as the mean±s.d. of four mice for each group. ***P<0.001, **P<0.01 and NS, not significant in Student’s t-test. (h) The expression levels of α germline transcripts (αGLT) in NALT CD45+ B220+ B cells prepared from WT mice at 5 days after vaccination. One representative experiment out of two is shown. ND, not detected. (i) The expression levels of Aicda mRNA in NALT CD45+ B220+ B cells prepared from WT or Ticam1−/− mice at 5 days after vaccination. Data are mean±s.d. of three independent samples. *P<0.05 in Student’s t-test.
As BAFF and APRIL on DCs are involved in the IgA CSR in the intestinal tract and lung,8, 15 we examined the expression of these molecules on CD103+ DCs. BAFF expression was higher in NALT CD103+ DCs than in CD103− DCs (Figure 9f). BAFF expression was upregulated after polyI:C treatment in WT mice but not in Tlr3−/− mice (Figure 9g). In contrast, APRIL expressed similarly between CD103+ and CD103− DCs (Figure 9f) and minimally induced by polyI:C in WT and Trl3−/− mice (Figure 9g). Hence, polyI:C mainly upregulates the expression of BAFF in CD103+ DCs in a TLR3-TICAM-1-dependent manner.
The expression of α germline transcripts, a maker of initiation of class switching from Cμ to Cα,7 was specifically detected in NALT B cells prepared from mice with i.n. inoculation with vaccine and polyI:C (Figure 9h). AID, which is an important factor for isotype switching in B cells,11 was expressed in NALT B cells 5 days after i.n. inoculation with polyI:C in WT mice, which was completely abrogated in Ticam1−/− mice (Figure 9i). Taken together, the TLR3–TICAM-1 axis in NALT governs TGF-β signal-mediated T helper 2 skewing to induce class switching after i.n. inoculation with vaccine and polyI:C. Our findings provide evidence that polyI:C induces various genes involved in the IgA CSR in NALT CD103+ DCs, resulting in the induction of AID expression to initiate Ig class switching in B cells.
Discussion
The purpose of this study was to clarify the mechanism by which polyI:C enhances vaccine-specific IgA production in the nasal cavity. CD103+ DCs are deeply involved in IgA production, which is followed by the T- and B-cell responses in a TLR3-TICAM-1-dependent manner. MAVS, IRF3, and IFNAR barely participate in IgA production. PolyI:C activates DCs through TLR3-TICAM-1 in NALT and allows B cells to induce the expression of AID via the DC/T-cell context. Expression of T helper 2–type cytokines and TGF-β-activating factors in NALT are crucial for TGF-β signaling in B cells, although the source of TGF-β in NALT cells remains unidentified. Moreover, CD103+ DCs expressing TLR3 in NALT increasingly liberate BAFF in response to polyI:C. PolyI:C directly activates B cells via TICAM-1 also, but this response is rather peripheral.
Although a part of IgG isotype Ab production at steady state is slightly impaired in Myd88−/−TrifLps2/Lps2, TLR-associated enhancement of Ab productions are still controversial in terms of MyD88 (myeloid differentiation primary response gene 88) and TRIF signaling.31 Furthermore, the signal pathway participating in sIgA response has remained unclear. This study sheds light on this issue: polyI:C-enhanced IgA production is clearly impaired in Tlr3−/− or Ticam1−/− mice but these knockout mice retain sufficient capacity for T- and B-cell activation in NALT. Type I IFNs enhance Ag-specific Ig production and cellular responses, thus having been used as a cytokine adjuvant.32, 33 However, the degrees of vaccine-specific Ig production in NALT appear to be similar in this study in Ifnar−/− and WT mice (Figure 2d) and impaired in Tlr3−/− mice irrespective of Ifn-β mRNA expression (Figure 9d), indicating that differential roles exist between the polyI:C and IFN therapy. PolyI:C-mediated T-cell activation occurs depending upon IRF3 unlike IgA production, suggesting the presence of IRF3-bypass pathway for Ig production in DCs. Ultimately, TLR3-TICAM-1 participates in T-cell activation and IgA production through two distinct pathways, IRF3/IFN and IRF3-independent axis.
The TLR3 pathway activates transcription factors, IRF3, NF-κB, and activator protein-1, which in turn induce type I IFN and inflammatory cytokines.5, 34 TICAM-1 specifically induces IL-1235 and activates TANK-binding kinase 1/I-kappa-B kinase ɛ and receptor-interacting protein 1/3 kinases in TLR3 signaling, which involves transcriptional activation of NF-κB and activator protein-1 for viral RNA-mediated host innate responses.21 Indeed, vaccine-specific Ig production in Irf3−/− mice is significantly elevated by polyI:C treatment (Figure 2d). IRF3-bypass induction of B-cell activation may happen in myeloid DCs in response to viral infection.36 Therefore, the activation of NF-κB and activator protein-1 contributes to polyI:C-mediated Ig production in addition to IRF3-mediated T-cell activation.
In accordance with previous studies,24, 37 Batf3−/− mice lacking CD8α+ and CD103+ DCs abrogate Ag-specific CD8+ and CD4+ T-cell responses in our study (Figure 6). As CD103+ DCs in the skin and lung tissue are able to present Ags to CD8+ T cells and CD4+ T cells,38, 39 NALT CD103+ DCs activated by polyI:C might also have the ability to prime CD8+ and CD4+ T cells. There are various DC subsets that sense viral RNAs in mice,40, 41 but their roles in Ig production have been poorly defined yet. Ag-specific Ig induction after vaccine and polyI:C treatment is abrogated in CD4+ T-cell-depleted mice (Figure 1e). Hence, polyI:C-enhanced Ig production requires the help of CD4+ T cells and entitles TD IgA production. The expression levels of genes involved in IgA induction, B-cell activation, and phosphorylation of Smad2 in NALT were decreased in polyI:C-treated Tlr3−/− mice compared with WT mice (Figure 9c,d and data not shown). Therefore, TLR3-dependent DC activation in concert with CD4+ T cells is induced by polyI:C and has an important role in TD IgA induction.
The first study reported using Batf3−/− mice show that Ag-specific IgG and IgM production is comparable with WT mice;24 but IgA production has not been examined. According to a recent study, IgA production in response to i.n. vaccination is impaired in Batf3−/− mice.15 Consistently, Batf3−/− mice exhibit strong suppression of polyI:C-mediated IgA production in our study, albeit subtle reduction of IgG production (Figure 6b). These results infer that NALT CD103+ DCs more potentially engage the production of IgA than IgG. The discrepancy between an earlier report24 and our present findings on IgG production in Batf3−/− mice remains unknown but possibly lies on differences in the setting, administration route, type of Ags, and genetic background of the knockout mice. Although Batf3−/− mice with the 129 background lack CD8α+ and CD103+ DCs, those with the C57B6/J background exhibit only partial depletion of CD8α+ and CD103+ DCs.42 Therefore, the remaining CD8α+ and CD103+ DCs in Batf3−/− mice with the C57B6/J background are likely to modulate immune responses induced by polyI:C. Although NALT CD103+ DCs upregulated IgA expression in B cells in vitro, IgA expression was also induced by NALT CD103− DCs (data not shown). Thus we do not exclude the possibility that TLR3-positive DCs other than CD103+ DCs are involved in IgA production.
The IgA CSR occurs in B cells in the GCs of secondary lymphoid organs and requires AID expression and requires cytokines, especially TGF-β, with or without the help of CD4+ T cells.7, 8 Previous reports show that the formation of GCs, AID expression, and an IgA CSR occur in NALT after i.n. inoculation of Ags.18, 43 Our findings are consistent with previous reports43 in that i.n. inoculation with polyI:C results in increased levels of AID expression in NALT B cells (Figure 9i). Although TGF-β signaling is a potent inducer of the IgA CSR in B cells,10 polyI:C did not directly induce Tgf-β1, 2, and 3 mRNA expression in NALT (Figure 9a). The results may reflect the posttranscriptional regulation of TGF-β activity.44 Activation of TGF-β is induced by its release from latency-associated protein, which in turn is promoted by αV integrin and MMP proteins, because TGF-β is produced as an inactive form, which can bind latency-associated protein.44 Although Tgf-β mRNA expression is unaffected in response to polyI:C inoculation, polyI:C enables NALT cells to upregulate TGF-β-inducible genes and phosphorylate Smad2 protein (Figure 9b,c). Because Peyer’s patch DCs and CD103+ DCs expressing Tgf-β mRNA are able to induce regulatory T cells in gut-associated lymph tissue,45 these DCs may produce TGF-β in gut-associated lymph tissue. Lung CD103+ DCs also produce TGF-β and induce IgA+ B cells.15 Yet, we could not identify cell types responsible for the supply of TGF-β or enzymes conversing to active forms in NALT.
Epithelial cells are an alternative source of active TGF-β.46 Because polyI:C did not directly induce TGF-β-inducible genes from the relevant DC subsets, such as splenic and bone marrow–derived DCs (data not shown), NALT epithelial cells may have a role in the production of TGF-β. In fact, polyI:C and influenza viruses evoke activation of TLR3 signaling and then TGF-β in epithelial cells.45, 46
I.n. administration of polyI:C and HA induces a high titer of anti-HA IgA2 and brings effective cross-protection against various H1N1 influenza virus challenge in mouse models. Here we show the mechanism by which polyI:C acts as an HA-specific IgA-producing adjuvant in nasal immunity. CD103+ DCs have a critical role via endosomal TLR3 and TICAM-1, rather than MAVS or IFNAR, in NALT IgA production; this DC subset transports influenza virus to the draining LNs in the lung, and mice were protected from infection by inducing effective cross-priming of CD8+ T cells.47, 48, 49 Likewise, NALT CD103+ DCs may co-operate with the respiratory system in provoking virus RNA-specific CD8+ T-cell responses.50 In either case, CD4+ T cells work for successful vaccination. TLR3 adjuvant makes appropriate cellular response against influenza throughout DC-enhanced events via NALT.
Methods
HA vaccine and vaccination
Split-product HA vaccine prepared from A/California/07/2009 (H1N1)pdm09 was gifted from Dr Y. Gomi in Research Foundation for Microbial Disease of Osaka University (Osaka, Japan). PolyI:C was purchased from GE Healthcare Japan (Tokyo, Japan). Mice were anesthetized with pentobarbital sodium and xylazine and i.n. immunized by dropping 5 μl phosphate-buffered saline containing 1 μg HA vaccine with or without 3 μg polyI:C or 1 μg CT (Wako Pure Chemical Industries, Osaka, Japan) as liquid drops into each nostril. Four weeks later, mice were re-immunized by the same method. Two weeks after second immunization, blood samples and NW fluid were collected from mice anesthetized by pentobarbital sodium and xylazine to measure vaccine-specific IgA and IgG Ab production. The levels of IgA and IgG Abs against HA were measured by ELISA as described previously2 using plates coated with HA protein purified from vaccine strain. Purified HA protein was gifted from Dr H. Hasegawa (National Institute of Infectious Disease, Tokyo, Japan). HA-coated plates were blocked with 1% bovine serum albumin/phosphate-buffered saline for 2 h and blocking buffer was discarded. Serum and NW were overlaid and then biotin-labeled goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) or IgA (Kirkegaard & Perry Laboratories, Gaithersburg, MD) were added. Streptavidin-alkaline phosphatase (Thermo Fisher Scientific, Waltham, MA) was reacted with biotin and detection was performed using p-nitrophenyl phosphate (Sigma-Aldrich, St. Louis, MO). The chromogen was measured by detection of absorbance at 405 nm using SUNRISE plate reader (TECAN, Mannedorf, Switzerland). For preparation of standard IgG in serum and IgA in NW, Balb/c mice were immunized as described above. To determine ELISA unit in standard samples, standard IgG in serum and IgA in NW were diluted in twofold steps from 1:200 to 1:6,553,600 for serum and 1:1 to 1:32,768 for NW with blocking buffer and performed ELISA as described above. The standard IgG in serum and IgA in NW had an ELISA end point titer of 1:409,600 and 1:2,048, respectively. These end point titers of each standard IgG in serum and IgA in NW were arbitrarily decided as 1 ELISA unit. Standard IgG in serum and IgA in NW were incorporated into each ELISA assay to make a standard curve. The IgG and IgA ELISA unit in test samples were calculated from their optical density at 405 nm values using the standard curve. Test serum samples and NW samples were diluted in 1:200 and 1:1, respectively.
For CD4+ T-cell depletion, mice were intraperitoneally injected with anti-murine CD4 Ab prepared from GK1.5 hybridoma before vaccination. Optimal doses of the Abs were determined in preliminary studies (150 μl per body). We confirmed a decrease in CD4+ T cells in the spleen, mesenteric LN and NALT. Detailed descriptions of the materials and methods used in this work are provided in Supplementary Materials and Methods.
Statistical analyses
Statistical significance of differences between groups was determined by the Student’s t-test, one-way analysis of variance test with Bonferroni’s multiple comparison post hoc test and Mann–Whitney U-test, using Prism 4 (GraphPad Software, San Diego, CA). Values of P<0.05 were considered significant.
References
Houser, K. & Subbarao, K. Influenza vaccines: challenges and solutions. Cell Host Microbe 17, 295–300 (2015).
Ichinohe, T. et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 79, 2910–2919 (2005).
Ichinohe, T., Ainai, A., Tashiro, M., Sata, T. & Hasegawa, H. PolyI:polyC12U adjuvant-combined intranasal vaccine protects mice against highly pathogenic H5N1 influenza virus variants. Vaccine 27, 6276–6279 (2009).
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 (2005).
Oshiumi, H., Matsumoto, M., Funami, K., Akazawa, T. & Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4, 161–167 (2003).
Matsumoto, M. & Seya, T. TLR3: interferon induction by double-stranded RNA including poly(I:C). Adv Drug Deliv. Rev. 60, 805–812 (2008).
Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 8, 421–434 (2008).
Bemark, M., Boysen, P. & Lycke, N.Y. Induction of gut IgA production through T cell-dependent and T cell-independent pathways. Ann. NY Acad. Sci. 1247, 97–116 (2012).
Litinskiy, M.B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002).
Coffman, R.L., Lebman, D.A. & Shrader, B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J. Exp. Med. 170, 1039–1044 (1989).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).
Tezuka, H. et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature 448, 929–933 (2007).
Tezuka, H. et al. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity 34, 247–257 (2011).
Fujimoto, K. et al. A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity. J. Immunol. 186, 6287–6295 (2011).
Ruane, D. et al. Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses. J. Exp. Med. 213, 53–73 (2016).
Kiyono, H. & Fukuyama, S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat. Rev. Immunol. 4, 699–710 (2004).
Liang, B., Hyland, L. & Hou, S. Nasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of mice. J. Virol. 75, 5416–5420 (2001).
Nacer, A. et al. Imaging murine NALT following intranasal immunization with flagellin-modified circumsporozoite protein malaria vaccines. Mucosal Immunol. 7, 304–314 (2014).
Errea, A., González Maciel, D., Hiriart, Y., Hozbor, D. & Rumbo, M. Intranasal administration of TLR agonists induces a discriminated local innate response along murine respiratory tract. Immunol. Lett. 164, 33–39 (2015).
Longhi, M.P. et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 206, 1589–1602 (2009).
Kawai, T. & Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 13, 460–469 (2007).
Desch, A.N. et al. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J. Exp. Med. 208, 1789–1797 (2011).
Edwards, A.D. et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33, 827–833 (2003).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Awe, O. et al. PU.1 expression in T follicular helper cells limits CD40L-dependent germinal center B cell development. J. Immunol. 195, 3705–3715 (2015).
Koyama, S. et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 179, 4711–4720 (2007).
Jenkins, G. The role of proteases in transforming growth factor-beta activation. Int. J. Biochem. Cell Biol. 40, 1068–1078 (2008).
Keeton, M.R., Curriden, S.A., van Zonneveld, A.J. & Loskutoff, D.J. Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor beta. J. Biol. Chem. 266, 23048–23052 (1991).
Skonier, J. et al. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol. 11, 511–522 (1992).
Spolski, R. & Leonard, W.J. Interleukin-21: a double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 13, 379–395 (2014).
Gavin, A.L. et al. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science 314, 1936–1938 (2006).
Proietti, E. et al. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J. Immunol. 169, 375–383 (2002).
Tovey, M.G., Lallemand, C. & Thyphronitis, G. Adjuvant activity of type I interferons. Biol. Chem. 389, 541–545 (2008).
Meylan, E. et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat. Immunol. 5, 503–507 (2004).
Matsumoto, M. et al. Defined TLR3-specific adjuvant that induces NK and CTL activation without significant cytokine production in vivo. Nat. Commun. 6, 6280 (2015).
Takaki, H. et al. MAVS-dependent IRF3/7 bypass of interferon β-induction restricts the response to measles infection in CD150Tg mouse bone marrow-derived dendritic cells. Mol. Immunol. 57, 100–110 (2013).
Mashayekhi, M. et al. CD8α(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity 35, 249–259 (2011).
Bedoui, S. et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10, 488–495 (2009).
Nakano, H. et al. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol. 5, 53–65 (2012).
Takaki, H. et al. The MyD88 pathway in plasmacytoid and CD4+ dendritic cells primarily triggers type I IFN production against measles virus in a mouse infection model. J. Immunol. 191, 4740–4747 (2013).
Takaki, H., Oshiumi, H., Matsumoto, M. & Seya, T. Dendritic cell subsets involved in type I IFN induction in mouse measles virus infection models. Int. J. Biochem. Cell Biol. 53, 329–333 (2014).
Tussiwand, R. et al. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature 490, 502–507 (2012).
Shikina, T. et al. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J. Immunol. 172, 6259–6264 (2004).
Massagué, J. & Chen, Y.G. Controlling TGF-beta signaling. Genes Dev. 14, 627–644 (2000).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Jolly, L. et al. Influenza promotes collagen deposition via αvβ6 integrin-mediated transforming growth factor β activation. J. Biol. Chem. 289, 35246–35263 (2014).
Helft, J. et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J. Clin. Invest. 122, 4037–4047 (2012).
Sung, S.S. et al. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 176, 2161–2172 (2006).
del Rio, M.L., Rodriguez-Barbosa, J.I., Kremmer, E. & Förster, R. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 178, 6861–6866 (2007).
Waithman, J. et al. Resident CD8(+) and migratory CD103(+) dendritic cells control CD8 T cell immunity during acute influenza infection. PLoS ONE 8, e66136 (2013).
Acknowledgements
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology, Research program on Emerging and Re-emerging Infection Diseases from Japan Agency for Medical Research and Development (AMED), Takeda Science Foundation, Uehara Memorial Foundation, Isukura, and the Akiyama Life Science Foundation.
Author contributions
T. Seya, H.T., and M.M. contributed to conception and design; H.T. and S.K. contributed to acquisition of data; H.T., T. Suzuki, A.A., H.H., H.O., and Y.S. contributed to the development of methodology; H.T., H.O., M.M., and T. Seya contributed to analysis and interpretation of data; H.T., H.O., and T. Seya contributed to writing the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
Supplementary Material is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Takaki, H., Kure, S., Oshiumi, H. et al. Toll-like receptor 3 in nasal CD103+ dendritic cells is involved in immunoglobulin A production. Mucosal Immunol 11, 82–96 (2018). https://doi.org/10.1038/mi.2017.48
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2017.48
This article is cited by
-
Evaluation of the TLR3 involvement during Schistosoma japonicum-induced pathology
BMC Immunology (2024)
-
Novel intranasal vaccine targeting SARS-CoV-2 receptor binding domain to mucosal microfold cells and adjuvanted with TLR3 agonist Riboxxim™ elicits strong antibody and T-cell responses in mice
Scientific Reports (2023)
-
Establishment of isotype-switched, antigen-specific B cells in multiple mucosal tissues using non-mucosal immunization
npj Vaccines (2023)
-
Towards the future exploration of mucosal mRNA vaccines against emerging viral diseases; lessons from existing next-generation mucosal vaccine strategies
npj Vaccines (2022)
-
Preparation and identification of monoclonal antibodies against porcine CD103
Applied Microbiology and Biotechnology (2022)