Abstract
Matrix protein 2 ectodomain (M2e) is considered an attractive component of a broadly protective, universal influenza A vaccine. Here we challenge the canonical view that antibodies against M2e are the prime effectors of protection. Intranasal immunizations of Balb/c mice with CTA1-3M2e-DD-generated M2e-specific memory CD4 T cells that were I-Ad restricted and critically protected against infection, even in the complete absence of antibodies, as observed in JhD mice. Whereas some M2e-tetramer-specific memory CD4 T cells resided in spleen and lymph nodes, the majority were lung-resident Th17 cells, that rapidly expanded upon a viral challenge infection. Indeed, immunized IL-17A−/− mice were significantly less well protected compared with wild-type mice despite exhibiting comparable antibody levels. Similarly, poor protection was also observed in congenic Balb/B (H-2b) mice, which failed to develop M2e-specific CD4 T cells, but exhibited comparable antibody levels. Lung-resident CD69+ CD103low M2e-specific memory CD4 T cells were αβ TCR+ and 50% were Th17 cells that were associated with an early influx of neutrophils after virus challenge. Adoptively transferred M2e memory CD4 T cells were strong helper T cells, which accelerated M2e- but more importantly also hemagglutinin-specific IgG production. Thus, for the first time we demonstrate that M2e-specific memory CD4 T cells are broadly protective.
Similar content being viewed by others
Introduction
The development of an effective universal, or broadly protective vaccine against pandemic influenza infections, is attracting increasing attention and several clinical trials have been completed or are underway.1, 2, 3, 4, 5 The medical and societal panic caused by the emergence of a pandemic H1N1 virus in Mexico in 2009 and the recurrent zoonotic infections with avian (H5N1, H7N9) influenza viruses, mainly in the far East, underscore the pressing need for a broadly protective influenza vaccine. Such a vaccine should comprise conserved influenza A virus epitopes that can be converted into safe and effective influenza vaccines that are easy to produce.6 However, the transformation of vaccine design strategies used for seasonal influenza virus vaccines into effective universal vaccines has proven difficult.7 For example, CD4 and CD8 T-cell responses against influenza viruses are broadly reactive and correlate with reduced disease severity in humans.8, 9, 10, 11 Despite this, it has remained a challenge to introduce influenza vaccines that also induce strong cellular responses. Rather, the traditional correlate of protection is based on hemagglutination inhibition IgG titers in serum and in the quest for a broadly protective influenza vaccine, researchers are combining multiple hemagglutinin (HA) subtypes or the relatively conserved stem domain of HA into a vaccine.12, 13, 14 Thus, there is still considerable confusion around which antigens to include, which adjuvants to use, and how these should be formulated and administered to obtain a broadly protective influenza vaccine.
A majority of the new influenza vaccine candidates are based on injections, whereas only few mucosal vaccines, in particular nasal or aerosol formulations, are being developed.15 This is unfortunate, because several studies have pointed to the critical cross-protective role of mucosal IgA and the importance of an effective tissue localization of resident memory T cells.16, 17, 18, 19, 20, 21, 22, 23 Only mucosal immunizations can effectively induce these responses. Moreover, the generation of virus-neutralizing IgA antibodies, to prevent influenza virus transmission, is best achieved following mucosal vaccination.20, 24
Conserved antigenic epitopes, from neuraminidase, nucleoprotein, or the HA stem, have been identified and evaluated for cross-protection against many subtypes of influenza A viruses.4, 12, 25 It is primarily serum antibodies that have been found to correlate with protection, although specific CD4 and CD8 T cells have a crucial role in cross-protection.8, 9, 10, 11, 26 Among the candidates for a universal influenza A vaccine is also the ectodomain of the matrix protein 2 (M2e).27 M2e is highly conserved in all human influenza A virus strains and has been found to be strongly immunogenic in various vaccine formulations and evaluated in clinical trials.28, 29, 30, 31, 32, 33 M2 is expressed in low copy numbers on the virion, but infected target cells abundantly express M2 on their surface where it is accessible for antibodies.30, 32, 34, 35 M2e-specific antibodies are not neutralizing. Rather, the protective mechanism of action for M2e-specific IgG antibodies depends on FcγR expression on alveolar macrophages and antibody-dependent cellular cytotoxicity or phagocytosis, which is similar to how anti-HA stem IgG antibodies protect.36, 37 The canonical view has been that protection by M2e-based vaccines correlates closely with the presence of anti-M2e antibodies, with high affinity for activating FcγR, and immunizations protect against multiple influenza A virus subtypes.38 We have previously reported on a broadly protective influenza A vaccine candidate based on M2e and the mucosal adjuvant CTA1-DD.39, 40 The latter is a non-toxic derivative of the cholera toxin A1 subunit and a dimer of fragment D from Staphylococcus aureus protein A.41 In particular, we were able to merge this mucosal adjuvant and M2e into a single immunoprotective mucosal vaccine formulation, the CTA1-3M2e-DD fusion protein. This fusion protein induced complete protection in mice following intranasal (i.n.) immunizations and both anti-M2e-specific antibodies and CD4 T-cell responses were induced.39 Whereas, much information is available on the immunogenicity and protective efficacy of M2e for stimulating antibody responses, very little has been published on the CD4 T-cell response to M2e. In fact, Gerhard and colleagues first described major histocompatibility complex (MHC) class II-restricted T-cell responses against M2e in 2003 and later Epstein and colleagues reported differential T-cell reactivity to M2e in different mouse strains.42, 43, 44 However, no detailed study on MHC class II-restricted M2e-specific epitope recognition or, more importantly, protective CD4 T-cell functions has previously been reported.
One line of research for the development of a universal vaccine is to explore how more potent CD4 and CD8 T-cell responses against conserved viral peptides could provide heterosubtypic protection.5, 8, 45 These strategies include the use of internal virus epitopes, such as those found in the viral nucleoprotein, matrix 1 protein, or polymerase subunits. Whereas protective CD8 T-cell immunity against heterosubtypic influenza strains has long been acknowledged, the protective role of CD4 T cells is attracting growing interest.10, 46, 47 Swain and colleagues have published an extensive literature on influenza-specific CD4 T-cell immunity and have shown that transfer of activated HA-specific CD4 T cells into naive hosts can protect against infection independently of B cells and CD8 T cells.48, 49 For protection, memory CD4 T cells could provide helper functions for influenza-specific CD8 T cells and activated B cells, but also other functions, including direct cytotoxicity as well as indirect effects on innate immunity in the lung have been proposed.
The CTA1-DD adjuvant has been proven effective at stimulating long-term memory and greatly augments antibody and cell-mediated immunity following mucosal and systemic immunizations.50 Given the broad protective role, CD4 T-cell epitopes may play in a universal vaccine and our earlier observation that M2e hosts a highly immunogenic class II-restricted CD4 T-cell epitope, we undertook this study to better define the protective function of CD4 T cells following i.n. immunizations with CTA1-3M2e-DD.
Results
Mapping of the M2e MHC class II-restricted T-cell helper epitope in CTA1-3M2e-DD
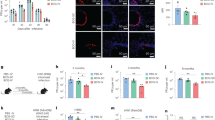
We have previously identified a MHC class II-restricted epitope in the influenza M2e peptide, which stimulated robust CD4 T-cell priming when combined with the CTA1-DD adjuvant in the fusion protein, CTA1-3M2e-DD.39, 40 In this study, we evaluated the precise role of M2e-specific CD4 T cells in influenza immunity. We performed a series of experiments that allowed us to map the M2e T-cell recognition epitope and to elucidate its possible mechanisms for protection. We found that Balb/c mice immunized three times i.n. with CTA1-3M2e-DD were fully protected against a potentially lethal challenge with X47 (H3N2) or PR8 (H1N1) virus (Figure 1a). Body weight loss and lung virus titers after X47 and PR8 challenge were significantly reduced in the CTA1-3M2e-DD group compared with control groups (Figure 1a and Figure 4b). MHC class II restriction was explored in three different inbred mouse strains and we found that, in contrast to Balb/c (H-2d) mice, C57BL/6 (H-2b) and DBA-1 (H-2q) failed to induce CD4 T cells specific for M2e (Figure 1b). The CD4 T-cell response to M2e in Balb/c mice was blocked by an anti-MHC class II H-2d Mab in a dose-dependent way (Figure 1b). Because the fusion protein carried multiple T-helper epitopes immunization of Balb/c and C57BL/6 mice resulted in comparable total serum anti-M2e antibody titers (Figure 1b). In DBA-1 mice, where these T-helper epitopes were not recognized, neither antibody nor M2e-specific CD4 T-cell responses were observed (Figure 1b). Challenging immunized DBA-1 mice with X47 virus resulted in weight loss and death in all mice, with significantly higher viral titers when compared with immunized Balb/c mice (Figure 1b). Interestingly, CTA1-3M2e-DD was fully adjuvant active as it promoted antibody production to co-administered tetanus toxoid, in all three mouse strains (Figure 1b). Hence, irrespective of whether CTA1-3M2e-DD was immunogenic or not, the adjuvant function was independent of MHC class II restriction. Priming with CTA1-3M2e-DD was dependent on dendritic cells (DC) as we observed significantly reduced responses in DC-depleted mice after diphtheria toxin treatment of CD11c-DTR mice (Figure 1c).51 Only low or undetectable levels of specific IFNγ or IL-17A production to recall antigen stimulation and strongly impaired anti-M2e antibodies were found in DC-depleted mice (Figure 1c).
MHC class II-restricted M2e-specifc immunity conveys cross-protection against influenza A virus infection. (a) Mice were immunized i.n. three times with CTA1-3M2e-DD (5 μg per dose), PBS or CTA1-DD (5 μg per dose) alone and challenged 6–8 weeks after the final dose with live virus at 4 × LD50 (lethal dose, 50%) with the mouse-adapted homologous X47 (H3N2) or the heterosubtypic PR8 (H1N1) strains. Mortality rates and body weight were monitored for 2 weeks after challenge. Lung virus titers were recorded 3 days after challenge with the PR8 strain and log10 values are given for TCID50 per lung (±s.d.). Mean weights shown where n>2. (b) CD4 T-cell responses to M2e are MHC class II restricted. Three different mouse strains were given three i.n. immunizations with CTA1-3M2e-DD admixed with 2 μg of TT; Balb/c H-2d (white), C57Bl7/6 H-2b (black), and DBA-1 H-2q (gray) and 7 days after the final dose mice were killed and serum and splenocytes were individually collected. CD4 T-cell proliferation was recorded in triplicates after 3 days of stimulation ex vivo with M2e peptide by 3H-thymidine incorporation and mean values are given as counts per minute (cpm)±s.d. MHC class II restriction of the M2e response in Balb/c CD4 T cells was confirmed by the blocking effect of a concentration range of anti-class II H-2d antibodies (black bars) or no antibody (gray bar) in splenocyte cultures and given as mean cpm±s.d. of triplicate cultures. Serum titers of total Ig anti-M2e were determined by ELISA and values are given as mean log10 titers±s.d. of five mice per group. (c) Priming with CTA1-3M2e-DD requires classical DCs as evidenced by lack of responsiveness after i.n. immunizations in CD11c-DTR chimeric mice. Experiments follow the protocols under a and b and results are given for DC-depleted (hatched) or WT control (black) mice and CD4 T-cell responses ex vivo after stimulation with CTA1-M2e (1 μM) are given as mean cpm± s.d., cytokine production is in pg ml−1±s.d. and mean and individual serum anti-M2e IgG log10 titers from five mice per group. The results in a–c are representative of three identical experiments giving similar results. Statistical significance was calculated using one-way ANNOVA (a and c) Mann–Whitney test (b); P<0.05 (*) and P<0.01 (**). (d) Amino acid (aa) sequences of long and short M2e-derived peptides to map the recognition sites of M2e by CD4 T cells. Proliferation of splenocytes from i.n., CTA1-3M2e-DD-immunized mice was determined with peptides at 3 μM and values are given as mean cpm±s.d. of triplicate cultures. (e) CD4 T-cell hybridomas were generated from i.n. immunized mice and two representative clones (28 and 40) are shown. M2e T-cell hybridoma clone 28 was used to screen for peptide recognition (IL-2 production) using the A20 B-cell line for I-Ad-restricted peptide presentation. Recognition was measured as mean cpm±s.d. of triplicates using the IL-2 responsive CTLL-2 cells. (f) A concentration range of the M2e peptides aa 1–16 and 7–17 were tested to determine the sensitivity of T-cell recognition (clone 28) and values are given as mean cpm±s.d. of triplicate cultures. Ovalbumin peptide p323-339 was used as negative control. (f) MHC class II restriction was assessed using a concentration range of blocking anti-I-Ad or I-Ed Mabs and A20 B cells presenting M2e peptide (3 μM) to the T-cell hybridoma clone 40 and values are given as mean cpm±s.d. of triplicate cultures. Panels d–f are representative of at least three experiments giving similar results.
We next used overlapping long and short peptides to identify the MHC class II H-2d-restricted epitope in M2e more precisely (Figure 1d). In splenocytes from mice that had been immunized with CTA1-3M2e-DD, a distinct CD4 T-cell recognition pattern was observed to recall peptide with amino acids (aa) 7–17, being the shortest peptide to stimulate a proliferative response, while aa 1–12, 1–14, 4–14, or 13–24 were not recognized and aa 10–20 only partly recognized (Figure 1e). Hence, the minimal peptide that was recognized corresponded to aa 7–17 of M2e (ETPIRNEWGSR). To facilitate further in vitro and in vivo analysis, we generated a set of M2e-specific CD4 T-cell hybridomas from immunized Balb/c mice. The M2e-specific CD4 T-cell hybridomas carried different Vβ TCR chains, Vβ4 or Vβ6, indicating that the response was oligoclonal (Figure 1e). These hybridomas recognized the M2e 7–17 peptide, but a peptide aa 1–16, was even better recognized, supporting that aa 7–13 and 15–16 were critical in the recognition of M2e (Figure 1e,f). Antibody-blocking experiments confirmed that the hybridomas were I-Ad restricted, while I-Ed was not involved in binding M2e (Figure 1f). Of note, the complete elimination of proliferation in the presence of anti I-Ad antibody confirmed our previous observation that there is no MHC class I-restricted CD8 T-cell epitope in the M2e peptide.39
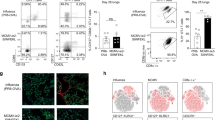
Based on the I-Ad restriction and the 1–16 aa peptide recognition, we generated MHC class II tetramers for the detection of M2e-specific CD4 T cells in vivo. Following i.n. immunizations of Balb/c mice with CTA1-3M2e-DD, we successfully detected such cells in spleen, lung, peripheral blood, and in the mediastinal (medLN) and cervical lymph nodes (CLN) (Figure 2a). Tetramer-specific splenocytes were restimulated with M2e peptide in vitro and the cytokine production was analyzed by FACS. Intracellular cytokine labeling of M2e-tetramer-specific CD4 T cells revealed four subtypes; IL-17A, IFNγ-, or TNFα-only producers or a multifunctional population that expressed IFNγ and TNFα (Figure 2b). Following challenge of immunized mice with influenza virus, these M2e-specific CD4 T cells increased significantly in all organs, with strongest expansion in the lung (30%) and medLN (2%). In contrast, no M2e-tetramer-specific CD4 T-cell response was observed in lungs of CTA1-DD immunized (not shown) or PBS control mice (0.2%) (Figure 2c). The M2e-tetramer-specific CD4 T cells were αβTCR (Figure 2d) and these cells carried multiple Vβ4, Vβ6, or Vβ8.3 chains (Figure 2e), supporting the notion that the response was oligoclonal. Of note, a substantial proportion of M2e-tetramer-specific CD4 T cells in the lung were RORγt+ (47%) and half of these produced IL-17A (50%), while none of the cells expressed granzyme B, suggesting that they were not cytolytic (Figure 2fFigure 2g).
In vivo distribution of M2e-tetramer-specific CD4 T cells following immunization and influenza A virus challenge. Balb/c mice were immunized i.n. three times with CTA1-3M2e-DD (5 μg per dose), PBS or CTA1-DD (5 μg per dose) and lymphocytes were isolated in single-cell suspensions from different organs (spleen, medLN, lung, cervical lymph nodes (CLN), and peripheral blood (PBL) before analyzing the presence of M2e-tetramer-specific CD4 T cells by flow cytometry (frequencies in upper right quadrant). Cells were labeled with the PE-conjugated M2e-specific class II tetramer, gated on 7AAD or Live/Dead Aqua negative (a–f), F4/80 negative (a–c,e), CD19 negative (a–c,e), C8a negative (a–f), CD4/M2e-tetramer double positive (f) (a) Distribution of M2e-tetramer-specific CD4 T cells in mice immunized with CTA1-3M2e-DD. Statistical significance was calculated using the unpaired t-test; P<0.01 (**). (b) Intracellular cytokine staining (ICS) after specific labeling of CD4 T cells following M2e peptide stimulation for 72 h in the presence of Brefeldin A for the final 6 h of culturing. ICS with anti-IL-17A, IFNγ, and TNFα antibodies conjugated with different fluorophores are shown. (c) Distribution of M2e-tetramer-specific CD4 T cells in various tissues in unimmunized (PBS) or immunized mice 4 days after a live X47 virus challenge infection (4 × LD50). CLIP-tetramer negative control labeling in isolated lung cells (right panel). (d) Surface TCR staining of M2e-tetramer-specific CD4 T cells from the lung of CTA1-3M2e-DD immunized mice 4 days after a live X47 virus challenge infection (e) Lung M2e-tetramer-specific CD4 T cells are oligoclonal following immunization with CTA1-3M2e-DD as determined by labeling with multiple TCR Vβ-specific Mabs. a–f are representative experiments of at least three giving similar results with three–five mice per group. (f) Co-expression of the transcription factor Ror-γt and IL-17A, as well as serine protease granzyme B by lung M2e-tetramer-specific CD4 T cells from CTA1-3M2e-DD immunized mice 4 days after a live X47 virus challenge infection. This is a representative experiment of at least three independent experiments giving similar results.
M2e-specific CD4 T cells provide significant protection against influenza A virus challenge
Antibodies are considered the prime mediators of protection in M2e-immunized mice, whereas the contribution of M2e-specific T cells remains largely elusive.38 Since M2e recognition by CD4 T cells was restricted to H-2d, and M2e-specific antibody responses were comparable between Balb/c and C57Bl/6 mice that had been immunized with CTA1-3M2e-DD, we analyzed to what extent M2e-specific CD4 T cells were critical for protection in congenic Balb/B (H-2b) mice. Balb/c and Balb/B mice were immunized i.n. with saline control, CTA1-DD alone or the fusion protein CTA1-3M2e-DD. M2e-specific serum IgG as well as IgG1 and IgG2a isotype titers were comparable in the two groups. In contrast to Balb/c mice, Balb/B mice were unable to fully control a virus challenge and less than 60% of these mice survived the infection, showing slower weight recovery and appearing to have higher viral titers (Figure 3a,b). Saline and CTA1-DD control immunized mice succumbed after challenge (Figure 3a). Post-challenge anti-M2e-specific IgG2a titers remained comparable between the two congenic mouse strains (Figure 3b). Prior to challenge, we observed strong splenocyte proliferation to recall stimulation with the CTA moiety of the fusion protein in both strains (Figure 3c). Responses to M2e peptide alone, however, were exclusively restricted to Balb/c mice, with prominent IFNγ and IL-17A production (Figure 3c). These findings suggested that M2e-specific CD4 T cells significantly contributed to protection and a failure to generate IL-17A and/or IFNγ may be responsible for the reduced protection. Importantly, Balb/B mice were able to generate functional helper CD4 T cells that could provide help to anti-M2e-specific B cells, because we found that cytokine mRNA and protein levels were upregulated in splenocytes from CTA1-3M2e-DD-immunized mice after ex vivo stimulation with the whole fusion protein (Figure 3d,e). In this setting, priming of Th1, Th2, Th17 as well TFH cells was confirmed in both Balb/c and Balb/B mouse strains, albeit with a clear skewing toward Th2 in Balb/c mice and a more prominent Th1 activity in Balb/B mice (Figure 3d). Thus, despite strong and comparable anti-M2e IgG1 and IgG2a serum antibody responses, M2e-specific CD4 T cells were critically required for full protection in this congenic mouse model.
Impaired protection against influenza infection in immunized mice in the absence of M2e-specific CD4 T cells. Groups of 10–12 Balb/c (H-2d) or congenic Balb/B (H-2b) mice were immunized three times i.n. with PBS, CTA1-DD, or CTA1-3M2e-DD (5 μg per dose). Six–eight weeks after the second boost, the mice were challenged with 4 × LD50 of X47 virus. (a) Survival and body weight loss was monitored for 2 weeks after challenge. Statistical comparisons between groups were done with the Mann–Whitney test (*: P<0.05). Lung virus titers were recorded 3 days after challenge with the X47 strain and log10 values are given for TCID50 per lung (±s.d.). (b) Anti-M2e serum IgG, IgG1, or IgG2a antibodies were determined 1 day before or 10 days after a challenge infection in immunized Balb/c (white) or Balb/B (black) mice. Values are given as individual and mean log10 titers for each group. (c) Splenocytes from unimmunized Balb/c and Balb/B (gray) or immunized Balb/c (white) or Balb/B (black) mice were cultured in triplicates with CTA or M2e peptide (1 μM) and supernatants were assessed for IL-17A or IFNγ production. Values are given as pg ml−1±s.d. a–c are from one representative experiment of three giving similar results. Statistical analysis used unpaired t-test; P<0.05 (*). (d) Cytokine gene expression profiles differ between CTA1-3M2e-DD immunized Balb/c and congenic Balb/B mice. Selected transcription factor and cytokine gene expression profiles of CD4 T-cell subsets to recall CTA1-3M2e-DD (1 μM) stimulation ex vivo using a PCR-array analysis of mRNA isolated from autoMACS-enriched CD4 T cells from the spleens of unimmunized or i.n. immunized Balb/c or Balb/B mice after 3–4 days after the second boost with CTA1-3M2e-DD. Individual samples were analyzed in triplicates from groups of five mice. The fold change, normalized against internal housekeeping genes in unimmunized mice, is given for the indicated cytokines. (e) Cytokine levels in the supernatants of the same samples as in d after stimulation of splenocytes with CTA1-3M2e-DD for 72 h with CTA1-3M2e-DD from naive (gray) or CTA1-3M2e-DD immunized Balb/c (white) or Balb/B (black) mice. The concentration of the indicated cytokines is given in ρg ml−1±s.d. d, e are from one representative experiment of two giving similar results.
M2e-specific CD4 T cells mediate protection in the complete absence of antibodies
To directly investigate the protective potential of M2e-specific CD4 T cells, we performed experiments in JHD mice, which lack B cells but have a functional T-cell immune compartment. Following i.n. immunizations with CTA1-3M2e-DD, M2e-specific CD4 T cells were similarly primed in JHD and Balb/c mice, and the production of M2e-induced IFNγ and IL-17A appeared comparable in the two strains (Figure 4a). Because B-cell-deficient mice are highly susceptible to influenza virus infection, we used a low challenge dose (corresponding to 0.2 × LD50 of X47 virus in the Balb/c model). We observed 50% survival in CTA1-3M2e-DD-immunized JHD mice, arguing that M2e-specific CD4 T cells can convey protection against infection in the complete absence of M2e-specific antibodies (Figure 4b). These mice also exhibited a significant reduction in lung virus titers, albeit not as pronounced as that observed in immunized wild-type (WT) mice, which were fully protected (Figure 4b). All unimmunized or CTA1-DD adjuvant alone immunized JHD mice succumbed to infection (Figure 4b). These results indicated that M2e-specific CD4 T cells were directly implicated in protection.
The M2e-specific CD4 T cells protect against infection in the complete absence of antibodies and protection partly depends on IL-17A. (a) Balb/c and B-cell-deficient JHD mice (Balb/c background) were immunized three times i.n. with CTA1-3M2e-DD (5 μg per dose). Seven days after the final dose CD4 T cells responses to recall M2e peptide (3 μM) stimulation in vitro were recorded in splenocytes and analyzed in triplicates for each mouse. Values for proliferation at 72 h after incorporation of 3H-thymidine are shown as mean cpm±s.d. for five mice per group. The supernatants of the M2e peptide stimulated cultures were analyzed for IFNγ and IL-17A production by ELISA and values are given as mean pg ml−1±s.d. (b) JHD and Balb/c mice were immunized three times i.n. with the indicated antigens. After 6–8 weeks, the mice were challenged with 0.2 × LD50 of X47 (H3N2) virus and monitored for survival. Lung virus titers were determined 6 days after challenge and log10 values are given as TCID50 per lung ±s.d. Statistical analysis used one-way ANNOVA P<0.05 (*). (c) CTA1-3M2e-DD-induced immunity is partly IL-17A-dependent. Balb/c (white) and IL-17A−/− (black) mice were immunized three times i.n. as indicated in a. After 6–8 weeks, the mice were challenged with 4 × LD50 of X47 (H3N2) virus and monitored for weight loss and survival. Lung virus titers were recorded 3 days after challenge and log10 values are given for PFU (±s.d.). Anti-M2e IgG antibodies in serum were determined before and after challenge and are shown as log10 titers in individual mice and mean values. (d) IFNγ-production to recall M2e stimulation was performed as in a in splenocytes isolated 7 days after the third i.n. immunization with CTA1-3M2e-DD of Balb/c and IL17−/− mice. These experiments are representative of three independent experiments giving similar results. Each group had 5–8 mice for immunogenicity studies and 10–12 mice for challenge studies. Statistical analysis was done using Mann–Whitney test and significance is indicated by P<0.05 (*) and P<0.005 (***).
Following i.n. CTA1-3M2e-DD immunizations, IL-17A production was prominent in M2e-tetramer-specific lung CD4 T cells (Figure 2b,e) and, therefore, we evaluated the effect of IL-17A for protection by comparing Balb/c and IL-17A-deficient mice (on a Balb/c background). Despite similar pre- and post-challenge, anti-M2e serum total IgG antibody titers, IL-17A-deficient mice exhibited significantly reduced protection, with slower weight recovery, significantly higher viral titers and only 50% survival, when compared with immunized WT mice (Figure 4c). Recall antigen stimulation of CD4 T cells revealed comparable IFNγ production, suggesting that IL-17A was critically required for full protection (Figure 4d). Thus, IL-17A and most probably Th17 cells have a key role in protection against influenza A virus infection in this model.
Memory M2e-specific lung-resident CD4 T cells convey protection
Protection against pandemic influenza virus strains induced by a broadly protective vaccine will most likely depend on strong immunological memory following immunization. Therefore, we analyzed to what extent we stimulated the presence of resident memory CD4 T cells in the lung following i.n. priming immunizations. We found significant numbers of CD62Llow CD69+ CD103low CD44+ M2e-specific memory cells in the lung 6 months after i.n. immunizations of Balb/c mice with CTA1-3M2e-DD (Figure 5a). These cells were truly lung-resident memory CD4 T cells because injecting labeled anti-CD45 Mab intravenously to the mice, as described by Anderson et al.,52 did not stain any M2e-specific CD4 T cells in the lung at 6 months after the i.n. priming immunization with CTA1-3M2e-DD (Figure 5b). Thus, according to previous publications and the definition of resident CD4 T cells, we concluded that i.n. immunization with CTA1-3M2e-DD effectively stimulated resident M2e-tetramer-specific memory CD4 T cells in the lung.
Intranasal immunization with CTA1-3M2e-DD stimulates M2e-specific lung-resident memory CD4 T cells that provide strong protection against live virus infection for more than 1 year. (a) Two months after i.n. immunizations of Balb/c mice with CTA1-3M2e-DD, we assessed the distribution of resident memory M2e-tetramer-specific cells to lung and other tissues (frequencies in upper right quadrant). Cells were labeled with PE-M2e-tetramer and various fluorophore-conjugated antibodies as indicated. (b) The resident CD4 T cells were identified as negative after i.v injection of APC-Cy7-labeled CD45.1 Mabs. (c) Balb/c mice were i.n. immunized and kept for 12 months before they were subjected to a live infection with 4 × LD50 of X47 (H2N3) influenza virus. Survival and weight loss were monitored in memory (CTA1-3M2e-DD) mice and unimmunized (PBS) age-matched control mice. Mean weights shown where n>2. Serum anti-M2e IgG log10 titers in memory mice before and after challenge are given as individual and mean values. Statistical analysis used one-way ANNOVA P<0.05 (*). (d) Expansion of M2e-tetramer-specific CD4 T cells (% of all CD4 T cells) in various tissues 1 year after i.n. priming immunizations and 4 days after a challenge infection with X47 (H2N3) influenza virus. a and c are representative dot-blots from two–three independent experiments. Gating was done on live cells for CD4 and PE-M2e-tetramer as shown and negative control, unimmunized (PBS) or CLIP-labeling, is shown (right 2 panels). (e) A distinct memory effector cell phenotype, CD44+, CD62Llow, and CCR7− was found in lung M2e-tetramer-specific CD4 T cells following challenge infection. ICS for IL-17A, IFNγ, and TNFα in lung memory M2e-tetramer-specific CD4 T cells. (f) Adoptive transfer of naive or M2e memory CD4 T cells into naive nu/nu (Balb/c) recipient mice. AutoMacs-enriched CD4 T cells were pooled from spleen, medLN, and CLN and 15 × 106 cells were adoptively transferred by i.v. injection. Frequencies of M2e-tetramer-specific CD4 T cells were assessed by FACS prior to transfer. (g) Nu/nu recipient mice demonstrated a dramatic expansion of memory M2e (upper panel), but not naive (lower panel), CD4 T cells in the lung and medLN after a challenge infection with 0.2 × LD50 X47 (H3N2) virus. Control CLIP-class II tetramer labeling (right panels). These are representative dot-blots from three independent experiments giving similar results with three nu/nu mice analyzed individually. The calculated expansion in absolute numbers of memory M2e-tetramer-specific (gray), as opposed to naive (white) CD4 T cells in various tissues in recipient nu/nu mice at 4 days after a live challenge infection.
We then waited 12 months before we evaluated whether the immunized Balb/c mice were protected against a live virus infection. Whereas immunized aged mice were fully protected against infection, age-matched unimmunized Balb/c mice succumbed after challenge (Figure 5c). Memory CTA1-3M2e-DD-immunized mice exhibited high levels of M2e-tetramer-specific CD4 T cells in the challenged lungs (17% of all CD4 T cells), in particular, but increases were also seen in medLN and spleen (Figure 5d). The lung CD4 T cells exhibited an effector memory phenotype, being CD44+, CD62Llow, and CCR7−, and 24% of these effector memory CD4 T cells produced IL-17A, while fewer produced IFNγ or TNFα (Figure 5e).
We estimated that we had a frequency of M2e memory CD4 T cells that was 0.3% of all CD4 T cells following i.n. priming immunizations. To assess the ability of the memory M2e-specific CD4 T cells to expand in a naive recipient host, we adoptively transferred 15 × 106 CD4 T cells pooled from the spleen, mediastinal lymph nodes (medLN), and CLN of memory mice (i.e., 1 year after CTA1-3M2e-DD immunization) to recipient nu/nu mice (Balb/c background). Based on M2e-tetramer staining, we estimated that approximately 45,000 M2e-specific memory CD4 T cells were present in the transferred CD4 T-cell pool (Figure 5f). Four days after a live virus challenge, these tetramer-specific cells expanded vigorously to frequencies ranging from 20 to 30% of all lung CD4 T cells and 1 to 4% in mLN and spleen (Figure 5g). This corresponded to roughly a 40-fold expansion. By contrast, mice that had received naive CD4 T cells exhibited negligible levels of M2e-tetramer-specific CD4 T cells after challenge (Figure 5g). Thus, memory M2e CD4 T cells demonstrated an exceptional ability to expand in the recipient host and migrated to the lung upon a challenge infection with influenza virus.
Memory M2e-specific CD4 T cells effectively accelerate isotype-switched responses in activated naive B cells
Although little is known about the requirements for an effective pandemic influenza vaccine, it is reasonable to assume that memory CD4 T cells could play a critical role.11 Lung-resident memory CD4 T cells could produce protective cytokines in situ, recruit effector memory CD4 and CD8 T cells to the lung or participate in direct killing of infected epithelial cells.48, 53 Alternatively, memory CD4 T cells could participate as TFH cells upon re-activation by antigen, supporting isotype-switching and affinity maturation of activated and proliferating primary B cells to produce more effective secondary type humoral responses.54 In immunized memory mice, but not in unimmunized control mice, we unexpectedly observed significant increases in serum anti-HA IgG antibody levels, suggesting that M2e-specific memory CD4 T cells provided secondary type TFH functions for naive HA-specific B cells (Figure 6a). By contrast, serum anti-HA IgM antibody levels were comparable in challenged mice, irrespective of whether they hosted naive or memory CD4 T cells (Figure 6a). Thus, it appeared that M2e-specific memory CD4 T cells could provide substantial B-cell help even to naive B cells, of unrelated specificity, such as HA-specific B cells. The latter indicated that naive B cells could function as antigen-presenting cells to memory CD4 T cells during an influenza virus infection. Using an influenza strain that expresses GFP,55 we found that 0.6% of lung B cells were GFP+, following a primary challenge infection, suggesting that such cognate antigen presentation to M2e memory CD4 T cells was indeed feasible for HA-specific B cells (Figure 6b).
Functional properties of TFH or Th17 subsets of M2e-specific memory CD4 T cells may explain the strong protection. (a) Balb/c mice were immunized i.n. with CTA1-3M2e-DD or unimmunized (PBS) and challenged after 12–18 months with 4 × LD50 of X47 influenza virus. Two weeks after challenge, serum IgM and IgG antibody responses to HA were assessed and mean log10 titers±s.d. are given for groups of 6–10 mice. (b) One unlabeled PR8 virus strain and one PR8 strain expressing GFP was used to assess if lung B cells were infected with/carried virus subsequent to a live challenge infection.55 We gated on B220+ IgD+ and IgD− B cells. (c) Adoptive transfer of naive or memory M2e CD4 T cells into naive nu/nu mice followed by a low-dose 0.1 × LD50 X47 (H3N2) influenza virus infection. Also in this model did memory M2e CD4 T cells support IgG-switched serum anti-HA and anti-M2e antibody responses, in particular, from activated naive B cells. (d) This was also manifest as greatly upregulated anti-M2e IgG or IgA responses in bronchioalveolar lavage (BAL) in these mice. (e) Viral load was assessed in lung and BAL on day 6 after challenge and values of TCID50 are given as mean log10±s.d. (f) Early neutrophil recruitment into the lungs 3 days after virus inoculation in naive or memory M2e CD4 T cells carrying mice. A representative FACS dot-blot shows dramatic recruitment of neutrophils in the lung and a calculation of Ly6G+CD11+ neutrophils (PMN) per 1,500 live leukocytes in BAL fluid is shown (right panel). These are representative experiments of three giving similar results with three–five mice per group and statistical analysis used one-way ANNOVA or unpaired t-test; P<0.05 (*).
To further dissect the TFH functions of M2e-specific memory CD4 T cells, we adoptively transferred these cells into naive recipient nu/nu mice and after a sublethal challenge infection with influenza A virus, we observed higher serum IgG anti-HA titers as well as significantly increased serum anti-M2e IgG antibody titers in mice reconstituted with M2e memory CD4 T cells as opposed to mice receiving naive CD4 T cells (Figure 6c). Importantly, mice receiving M2e memory CD4 T cells exhibited significantly reduced viral load in the lungs and BAL, in particular (Figure 6d). The latter was associated with a significant anti-M2e IgA and IgG response in BAL, suggesting a protective role of mucosal antibodies (Figure 6e). In addition, a dramatic increase of lung neutrophils was observed already 2 days after a viral challenge in mice adoptively transferred with memory M2e-specific CD4 T cells (Figure 6f). Taken together, these results argue that memory CD4 T cells against M2e can convey strong resistance against influenza virus infection in multiple ways. This could be achieved by orchestrating direct and indirect protective measures, including neutrophil recruitment to the lung and promoting isotype-switched systemic and mucosal antibodies of multiple specificities from naive B cells, including anti-HA antibodies. Thus, memory M2e CD4 T cells appear to exert direct multifunctional effects as lung-resident and migrating effector CD4 T cells, producing IL-17A and IFNγ (and perhaps other cytokines) as well as functioning as effective TFH cells supporting priming and differentiation of isotype-switched B cells in draining lymph nodes. Knowing that M2e has been found to stimulate strong protection against multiple influenza A virus subtypes and strains, we propose that a mucosal vaccine based on M2e memory CD4 T cells could be a way forward in the design of broadly protective vaccines against pandemic influenza infections.
Discussion
Broadly protective immunity against influenza can be provided by cross-protective antibodies and memory CD4 and CD8 T cells.8, 14, 31, 48, 56 Memory CD4 T cells are at the center of the adaptive immune response, because they provide helper functions to B and CD8 T cells, regulate innate immunity and can directly combat pathogens.11, 48 Hence, it is likely that a broadly protective vaccine against pandemic influenza will rely on the induction of long-lasting cross-protective CD4 T cells. Efforts to understand CD4 T-cell protective functions and recognition of conserved influenza antigens are therefore crucial for the development of a broadly protective influenza vaccine.
The present study is the first to investigate in detail the protective function of CD4 T cells specific for M2e, which is, perhaps, the most explored candidate antigen for a broadly protective influenza virus vaccine.28, 38, 42 We found that i.n. immunizations of Balb/c mice with CTA1-3M2e-DD-stimulated memory M2e-specific CD4 T cells that critically protected against infection. Using specific tetramers, we could follow the accumulation and expansion of these CD4 T cells in the lung upon i.n. immunizations and after a viral challenge infection. M2e-specific CD4 T cells were found to be critical for protection in the complete absence of antibodies (JHD mice), or when high levels of anti-M2e IgG2a antibodies, but no M2e-specific CD4 T cells were present, as in immunized congenic MHC class II mismatched Balb/B mice. Whereas a proportion of the lung CD4 T cells were multifunctional and made IFNγ and TNFα, as many as 50% of the M2e-specific CD4 T cells in the lung were Rorγt+ Th17 cells, of which half produced IL-17A. Since IL-17A−/− mice exhibited a significantly reduced level of protection despite high levels of M2e-specific IgG2a antibodies and IFNγ production, we concluded that IL-17A appeared to exert a critical protective function. This could, in part, be explained on the basis of an early influx of neutrophils after a virus challenge infection as seen in mice hosting resident M2e memory CD4 T cells.57 Alternatively, these memory CD4 T cells could be cytolytic.53 However, this was not supported by the complete lack of granzyme B in the M2e-tetramer-specific lung CD4 T cells after a challenge infection. Furthermore, we found that not only pre-existing M2e-specific B cells but also naive B cells, such as those responding to the unrelated HA protein, underwent enhanced switching to IgG in the presence of M2e-specific memory CD4 T cells. Although previous findings identified that priming of T cells with conserved antigens of influenza virus could lead to an increased ability to boost antibody responses to hemagglutinin, the mechanism for such an effect was not determined.58 However, our observation suggested that activated B cells were infected or carried influenza virus antigens that, including M2e in a cognate fashion, activated the M2e-specific memory CD4 T cells to generate a more efficient isotype-switched, antibody response. Noteworthy, cognate help for IgG-switched anti-HA and anti-M2e primary antibody production also appeared prominent in naive nude mice adoptively transferred with M2e-specific memory CD4 T cells. Indeed, evidence that lung B cells can be infected or take up virus was found using a GFP-expressing PR8 strain.55 Whether or not this observation sufficiently explains how cognate interactions can occur between naive HA-specific B cells and memory M2e CD4 T cells is too early to conclude. In fact, an enhanced antibody response to virus after transfer of memory CD4 T cells to naive recipient mice, subsequently challenged with virus, has been reported previously by both Swain’s and Gerhard’s groups.59, 60 In particular, the latter group used nu/nu mice as recipients of CD4 T-cell clones and they showed protection associated with antibody production, but only against viruses that carried the MHC class II-restricted epitopes.60 However, these studies did not determine isotype-switched antibodies or the actual mechanism behind the antibody production, whether cognate or non-cognate in nature. On the other hand, researchers have also documented that naive B-cell responses may be modulated to express protective IgA anti-influenza antibodies as a consequence of non-cognate interactions.61 In such a non-cognate case, activated lung memory CD4 T cells do not have to recognize M2e on the B cells to support isotype-switched anti-HA antibodies. This type of modulation would probably be extra-follicular in nature, while modulation of the germinal center reaction traditionally has required cognate interactions between activated B and T follicular helper (Tfh) cells.54 Ongoing studies in our laboratory are attempting to address the question of cognate or non-cognate memory CD4 T-cell help to naive B cells in influenza virus infections. It has previously been observed that memory CD4 T cells are effective at accelerating B-cell help.62 Indeed, our finding confirms that memory CD4 T cells are effective at accelerating B-cell help and it underscores a critical function of MHC class II-restricted conserved epitopes in a broadly protective influenza vaccine.62 This way, M2e-specific memory CD4 T cells could facilitate isotype-switched neutralizing and non-neutralizing antibody production to many influenza antigens, including HA-specific antibodies, which are critically important for protection against infection.
In agreement with previous studies of heterosubtypic protection, we found memory CD4 T cells to be critical.63, 64, 65, 66, 67, 68While this is the first study to focus on M2e-specific CD4 T cells, other researchers have documented how HA-specific CD4 memory T cells can transfer protection.23, 48 In both these studies, IFNγ was found to be important for protection as neutralization of IFNγ with antibody abrogated protection. However, protection against influenza could also be independent of IFNγ through direct cytolytic functions, which appeared to be granzyme B-dependent and MHC class II restricted.69 However, our M2e-tetramer-specific CD4 T cells in the lung did not express granzyme B, and, hence could not exert a cytolytic function. To what extent the viral challenge infection elicited enhanced CD8 T-cell responses to HA or nucleoprotein or other internal antigens following CTA1-3M2e-DD immunization was not investigated. Although M2e does not carry a CD8 T-cell epitope, the presence of memory M2e-specific CD4 T cells in the host could potentially influence the priming of multiple other CD8 T cells during infection. This way, not only M2e-specific CD4 T cells would be critical in our model, but also CD8 T cells recognizing internal epitopes could be promoted. Future studies will explore this possibility.
Memory CD4 T cells are characterized by rapid expansion and production of cytokines upon activation and in this capacity they are thought to effectively convey resistance against infection.70, 71 We found robust expansion of M2e-specific memory CD4 T cells—at least 40-fold—after transfer into nu/nu mice, and significant IFNγ-production, which in some cells was co-produced with TNFα.72 However, we also observed that IL-17A producing M2e-specific CD4 T cells were prominent in the lungs of immunized and infected mice and 50% of these cells expressed the master gene regulator Rorγt, associated with Th17 cells.73 Moreover, when nasal immunizations were performed in IL-17A−/− mice, we observed a dramatic reduction in protection (50% survival) against infection, despite comparable M2e-specific IFNγ production by CD4 T cells and anti-M2e-IgG serum antibody levels as seen in WT mice. Thus, M2e-specific CD4 T-cell protection appeared to be partly dependent on IL-17A and, probably Th17 cells. This conclusion is, however, at variance with a previous study, which showed that Th17 polarized HA-specific CD4 T cells could mediate protection against influenza infection, but in an IL-17A-independent fashion.59 Also, these authors reported that the Th17-mediated protection was under IL-10 regulation and was observed in IL-10−/− mice, but it was not prominent in WT mice. Following adoptive transfer of M2e memory CD4 T cells into nu/nu hosts, we found significantly reduced viral load in BAL/lung at an early time point after a viral challenge infection. This was associated with an influx of neutrophils in the lungs, which could have been the result of enhanced IL-17A production.57
The mechanism for IL-17A-mediated protection, however, is poorly understood at present. Several studies have described protection associated with Th17 activity not only against influenza, but also against tuberculosis, chlamydia, and pertussis.74 Hence, IL-17A could convey resistance to infection, but it could also exacerbate inflammation, which could be detrimental and cause lung injury in response to influenza virus infection.75, 76 Additional studies are required to resolve why our study with M2e-specific CD4 T cells demonstrated prominent protection associated with IL-17A formation, whereas other investigators have identified little protective capacity of IL-17A against influenza virus infection.59, 76 Although M2e-specific CD4 T cells were αβ TCR+ cells, IL-17 production in the context of influenza virus infection could be produced by γδTCR+ cells.77 It has been suggested that susceptibility to secondary bacterial infection following primary influenza disease may result from negative regulation of γδ T cells, so generation of IL-17 by an alternative subset may play a role in reducing influenza-associated morbidity and mortality.78 A recent publication describes a fascinating system by which influenza-specific CD8+ T cells in the airways are guided by CXCL12 trails produced by neutrophils in the airways.79 In accordance, resident M2e-specific CD4 T cells could recruit neutrophils by IL-17A production in the lung enabling CD8 T cells to protect against infection. Moreover, the presence of newly formed lymphoid tissues in the lung, so called induced bronchial-associated lymphoid tissue (iBALT), is also critically dependent on IL-17.80 iBALT formation has been associated with elimination of virus-infected cells and, hence, the consequence of a lack of IL-17 could be poor virus elimination in the lung.81, 82 Alternatively, lung-resident Th17 cells and levels of IL-17 have been linked to lung IgA production, supporting our finding that adoptively transferred M2e-tetramer-specific memory CD4 T cells do, indeed, promote significant anti-M2e IgA antibodies in BAL, in naive nude mice.83 Nevertheless, it should be emphasized that using the CTA1-3M2e-DD self-adjuvanted fusion protein, we successfully generated lung-resident, memory Th17 cells that upon a heterosubtypic virus challenge infection effectively protected against morbidity and mortality. While we achieved this effect with an adjuvanted fusion protein, Zens et al.84 recently failed to stimulate lung-resident, memory CD4 T cell unless a live attenuated influenza vaccine was administered i.n. Therefore, we can conclude that the i.n. route is critical for generating lung-resident CD4 T cells, while also adjuvanted killed vaccines can be effective, contrary to what these authors proposed.
A cardinal feature of vaccine-induced M2e immunity is that it allows for the generation of a primary humoral and cell-mediated immune response to an influenza challenge infection.82 This is in line with the notion that a vaccine against heterosubtypic influenza infection does not need to stimulate sterile immunity, but rather should allow for transient infection to revoke memory CD4 and CD8 T-cell immunity. Thus, an M2e-based vaccine cannot be assessed on the basis of neutralizing antibodies, but new correlates of protection need to come into place. Protection by our vaccine approach relies on the presence of memory M2e-specific CD4 T cells. These recognize a CD4 T-cell epitope that minimally is restricted by aa 7–17, in which aa 15 and 16 are critical, since aa 1–14 could not be recognized by M2e-specific CD4 T cells. The same sequence has been found to be recognized by human CD4 T cells associated with protection.10 Interestingly, a B-cell epitope in M2e includes this range of aa 7–17, but it appears that differences in aa at certain positions 13, 14, or 18 between different strains do not markedly affect the protective efficacy of M2e immunization, whereas mutations in aa 10, 11, or 16 reduce the antibody-binding affinity.85, 86, 87 Considering that M2e-specific CD4 T cells are also protective, some earlier attempts at correlating protective efficacy following M2e immunization to antibody-binding specificities exclusively may have to be revisited. A recently published report from the World Health Organization Product Development for Vaccines Advisory Committee identified 41 universal influenza vaccine candidates at various stages of development; a list that includes our own CTA1-3M2e-DD.88 Of those 41 candidates, 10 utilize M2e (6 pre-clinical, 3 phase one, and 1 phase two). In light of our findings, and with one quarter of universal vaccine candidates utilizing M2e, it is clear that in addition to anti-M2e antibody, standardized tools to evaluate and compare cellular responses to differently adjuvanted M2e-based vaccines are much warranted.
It is likely, in fact, that the CD4 T-cell epitope is functionally more conserved than the B-cell epitope in M2e and, therefore, also less susceptible to mutations. A tetrameric M2e vaccine formulation has been found to enhance anti-M2e antibody responses, but it would not necessarily be required for the generation of protective memory CD4 T cells, for which a less sophisticated vaccine based on peptides or monomers would be sufficient.85, 86, 87
The CTA1-DD adjuvant has previously been shown to be non-toxic in mice and monkeys, it does not drive inflammation at the site of application and, importantly, it does not bind GM1-ganglioside receptors or accumulate in the central nervous tissues following i.n. administration as cholera toxin and other holotoxin adjuvants do.89, 90, 91 Hence, it should be a safe and clinically attractive mucosal adjuvant.41 Moreover, the CTA1-DD adjuvant greatly augments the germinal center reaction and was proven a strong inducer of immunological memory in both CD4 T cells and B cells.50 We believe the CTA1-3M2e-DD construct complemented with additional epitopes could be a feasible universal influenza vaccine candidate.
Methods
Preparation of the fusion protein
We expressed and purified the CTA1-3M2e-DD fusion protein essentially as described.39 Briefly, the purified material contained no endotoxin (<1EU per mg of protein), it bound avidly to IgG in solid phase and it was enzymatically active (CTA1), effectively ADP-ribosylating agmatine.39
Mice
Balb/c and congenic Balb/B (C.B10-H2b/LilMcdJ) mice were obtained from Harlan (Kreuzelweg, The Netherlands) and Charles River (Sulzfeld, Germany). C57Bl/6, DBA-1 and nude (nu/nu) mice (on a Balb/c background) were obtained from Taconic (M&B, Lille Skensved, Denmark), Jackson and Harlan (Netherlands), respectively. B-cell deficient (JHD), IL-17A−/−, and CD11c-DTR transgenic (B6.FVB-Tg Itgax-DTR/GFP 57Lan/J),51 all on Balb/c background, were bred and maintained under specific pathogen-free conditions at the Laboratory for Experimental Biomedicine (EBM), University of Gothenburg (Gothenburg, Sweden). Experiments were undertaken with ethical permission from local authorities.
Immunizations and influenza challenge infections
Five to 15 age- and sex-matched mice per group were immunized intranasally (i.n.) three times, 10 days apart with 20 μl PBS or PBS containing indicated doses of CTA1-3M2e-DD (carrying three copies of the M2e peptide) or CTA1-DD adjuvant alone. As an unrelated antigen, we admixed tetanus toxoid (TT) (Statens Serum Institute, Copenhagen, Denmark) at 2 μg per dose. Immune responses were evaluated 6–8 days after the final immunization or as indicated. Unimmunized and immunized mice were challenged with live influenza A virus after 4–8 weeks and experiments were undertaken at EBM (University of Göteborg) or at the Department of Molecular Biology, VIB, (Ghent, Belgium).39 Briefly, we used a potentially lethal dose (4 × LD50, corresponding to 2.5 × 103 TCID50) of the mouse-adapted homologous X47 virus (H3N2) or the heterosubtypic PR8 (H1N1) strain administrated i.n. to mice under light anesthesia. Weight loss and mortality was monitored on a daily basis for 2 weeks. According to the ethical permit, mice were killed when a loss of body weight exceeded 25% of total body weight. When indicated, we used the lower challenge dose of 0.2 × LD50 of X47 virus for experiments in B-cell deficient (JHD) and nu/nu mice.
Determinations of antibody responses
Serum or bronchial lavage (BAL) samples were collected from individual mice at indicated time points. Antibody determinations were performed by ELISA using microtiter plates (MaxiSorp, Nunc) coated with 100 μl of 2.5 μg ml−1 M2e peptide in 50 mM PBS, pH 9.7, or 2 μg ml−1 TT in PBS and incubated overnight at +4 °C. After blocking with PBS with 0.1% BSA, serial 1:3 or 1:5 dilutions of the samples were performed starting with 1/25–1/50 dilution. Bound antibodies were detected with alkaline phosphatase-conjugated antibodies directed against mouse IgG, IgG1, or IgG2a diluted 1/1,000 (Southern Biotechnology, Birmingham, AL) and after incubation followed by nitrophenyl (NPP) phosphatase substrate (1 mg ml−1, SIGMA) in ethanolamine buffer, pH 9.8. Absorbance was measured at 405 nm using a Multiscan MS spectrophotometer. The linear part of the curve was used for calculating titers at a cutoff level of 0.4 above background. Values are given as mean log10 titers±s.d. HA-specific antibody responses were determined as follows: 96-well microtiter plates were coated overnight at 4 °C with 50 μl of supernatant derived from HEK293T cells that had been transfected with a pCAGGs expression plasmid that encodes secretory X47 HA. After washing and blocking with 1% BSA/PBS serum, samples were diluted 1/100 and then threefold serially diluted in subsequent subwells. Bound antibodies were detected using HRP-conjugated secondary antibody, followed by addition of TMB substrate. The reaction was stopped by adding 50 μl H2SO4 (1 M) to each well and the OD450 in each well was measured with an ELISA reader. End point titers are defined as the highest dilution resulting in an O.D. value twice that of the background value (pre-immune serum).
Determinations of CD4 T-cell immunity
Lymphocytes were isolated from the spleen, cervical lymph nodes (CLN), mLN, or lung of immunized mice. Briefly, cells from spleen and mLN were isolated by mechanical disruption. Lung tissues were enzymatically digested at 37 °C for 90 min with Roswell Park Memorial Institute (RPMI) containing collagenase (4,000 IU ml−1), DNAse (1 mg ml−1), horse serum (10%), and antibiotics followed by lysis of red blood cells and an additional incubation in Petri dishes for 90 min at 37 °C with RPMI alone to eliminate adherent cells. Alternatively, single-cell suspensions were prepared from lungs with a commercially available kit from Miltenyi Biotec (Bergisch Gladbach, Germany) (ref 130-095-927). Splenocytes were cultured in 96-well microtiter plates (Nunc) for 3 days alone or with different concentrations of recall antigen to stimulate ex vivo CD4 T-cell proliferation and cytokine production. Proliferation was determined using a beta scintillation counter (Beckman Coulter, Turku, Finland) after addition of [3H]Thymidine (Amersham Bioscience, Uppsala, Sweden) for the final 6 h of culturing. Supernatants were collected and stored at −70 °C until further analyzed. Cytokine production was determined using ELISA (Duoset, R&D Systems, Abingdon, UK) and the concentrations of IFNγg, TNFα, and IL-17A in supernatants were expressed in ρg ml−1.
Adoptive transfer of memory CD4 T cells
Enriched memory CD4+ T cells from mice immunized 12–18 months earlier were collected and pooled from spleen, CLN, and mLN by positive magnetic separation using MACS separation column (Miltenyi Biotec). Briefly, a single-cell suspension was prepared and CD4+ cells were sorted using the Miltenyi CD4 T-cell isolation kit as per the manufacturer’s instructions. Pooled CD4+ T cells, at 10–15 × 106 cells, were injected intravenously into recipient nu/nu mice (Balb/c). The purity of transferred CD4+ T cells was >90% as determined by FACS. Reconstituted recipient mice were challenged 1 day after the transfer with low-dose live influenza X47 virus i.n. and mice were monitored for disease symptoms.
Bone marrow chimeras and DC depletion model
Femurs and tibias were taken from donor CD11c-DTR mice and bone marrow was flushed out. Cells were passed through a 70-μm nylon mesh and red blood cells were lysed. CD11c-DTR bone marrow donor cells were used to generate CD11c-DTR→WT chimeras (CD11c-DTR/WT).51 C57BL/6 recipient mice were irradiated (1,000 rad) and 2.5–5 × 106 bone marrow cells were injected intravenously Mice were rested for 6 weeks prior to DC depletion by injecting 4 ng g−1 body weight of diphtheria toxin (Sigma-Aldrich) 1 day before start of immunizations.
Generation of M2e-specific T-cell hybridomas
Spleens were collected from mice 14 days after immunization with CTA1-3M2e-DD. Following red blood cell lysis and washing the CD4 T cells were activated in vitro with 1 μM M2e peptide for 2 days in complete DMEM. CD4 T-cell hybridomas were generated using a standard protocol with BW5147 fusion partner cells in polyethylene glycol 1500.92 Following fusion, cells were distributed into 96-well flat-bottom culture plates (Nunc, Roskilde, Denmark) and hybridomas were selected in medium containing hypoxanthine–aminopterin–thymidine and expanded into 24-well plates. After 72 h, irradiated splenic feeder cells were added to the CD4 T-cell hybridomas and cultures were stimulated with M2e peptide or TT, as a negative control, and supernatants were scored for IL-2 production using the CTLL-2T cell line. Hybridomas testing positive for M2e peptide recognition and negative for TT antigen were selected and further expanded and subcloned by limiting dilution.
Synthetic peptides, MHC class II blocking antibodies, and M2e tetramers
We designed shorter and longer peptides with overlapping aa sequences covering the entire 1–24 aa long M2e peptide of the X47 H3N2 influenza A virus (PEPSCAN, Leylstad, Holland). All overlapping peptides were tested at indicated concentrations ranging from 0.01 to 3 μM together with CD4 T cells from CTA1-3M2e-DD-immunized mice or the M2e-specific T-cell hybridomas that we generated. For MHC class II blocking experiments, we used specific H-2d-, I-Ad-, or I-Ed-specific antibodies or isotype control antibodies (BD Biosciences, San Jose, CA). Based on the optimal peptide recognition requirement of aa 1–16, the NIH Core Tetramer Facility generated M2e-specific MHC class II-restricted (I-Ad) tetramers (Emory University, Atlanta, GA).
Flow cytometry
Flow cytometry analysis was performed on a BD LSR II instrument (BD Biosciences) and results were analyzed using FlowJo software (Tree star, Ashland, OR). We used 7-amino-actinomycin D or live/dead Fixable Dead Cell Stain Kit (Invitrogen, Renfrewshire, UK) to exclude dead cells. The following fluorophores and conjugated antibodies were used for phenotypic analysis; tetramer-PE, CLIP-PE (negative control) and CD4 Alexa-700. CD8 FITC or AF647, CD19-FITC and F4/80 FITC were used for a dump channel. For labeling memory CD4 T cells, we used CD4 BV786, CD62L APC, CD69 APC-Cy7, CD103 BV421, and CD44 AF700. Intracellular staining for cytokines was performed after stimulating CD4 T cells with PMA and ionomycin for 4 h in the presence of Brefeldin A (BD Biosciences), before labeling with combinations of the following: CD4 AF700, TCRβ PE-Cy7, ROR-γT PE-CF594, IL-17A FITC, IFNγ BV605, TNFα PE-Cy7, and granzyme B eFluor450. For detection of neutrophils, we used labeled Ly6G and CD11b Mabs (BD Biosciences). Of note, M2e-specific tetramer staining was performed at 37 °C for 30 min followed by labeling with other surface markers on ice for 30 min. Antibody panels to screen for mouse Vβ T-cell receptors were used (mouse Vβ TCR screening panel from BD Pharmingen, San Diego, CA). For detection of influenza-infected lung B cells, we used the GFP+-influenza-infection system, recently described.55 Briefly, 4 days following inoculation with the virus, lung cells were isolated and B cells were identified by sorting on a BD AriaIII equipped with red, blue, yellow–green, and violet lasers to optimize separation between GFP-positive and -negative cells and using antibodies recognizing B220 APC, IgD BV510, NK1.1 biotin, CD3 biotin CD11b biotin, and CD11c biotin followed by subsequent addition of streptavidin APC-Cy7 (BD Biosciences).
DNA and RNA extraction from sorted cells and cDNA synthesis
Highly purified CD4+ T cells from CTA1-3M2e-DD-immunized Balb/c or congenic Balb/b mice were pelleted and RNA was extracted using RNA-easy mini kit (Qiagen, Hilden, Germany). For cDNA synthesis, 1 μg RNA was used together with oligo-(dT) and random primers (QuantiTect Reverse Transcription, Qiagen). The cDNA synthesis was carried out at 42 °C for 15 min, followed by 3 min at 95 °C to inactivate the enzyme. cDNA was further analyzed using a PCR-array kit (Th1-Th2-Th3) in 96-well plates according to the manufacturer’s instructions (SABioscience, Qiagen).
Study approval
This study was approved by the local ethics committee no: UGOT 12–31.
Statistics
Analysis of significance was done in Prism (GraphPad Software) using Mann–Whitney test, one-way ANOVA, or unpaired t-test, as indicated. All reported P-values are two-sided and values of less than 0.05 were considered to indicate statistical significance. P<0.05 (*), P<0.01 (**), and P<0.005 (***).
References
de Vries, R.D., Altenburg, A.F. & Rimmelzwaan, G.F. Universal influenza vaccines, science fiction or soon reality? Expert Rev. Vaccines 14, 1299–1301 (2015).
Gerhard, W., Mozdzanowska, K. & Zharikova, D. Prospects for universal influenza virus vaccine. Emerg. Infect. Dis. 12, 569–574 (2006).
Houser, K. & Subbarao, K. Influenza vaccines: challenges and solutions. Cell Host Microbe 17, 295–300 (2015).
Soema, P.C. et al. Current and next generation influenza vaccines: formulation and production strategies. Eur. J. Pharm. Biopharm. 94, 251–263 (2015).
Zheng, M., Luo, J. & Chen, Z. Development of universal influenza vaccines based on influenza virus M and NP genes. Infection 42, 251–262 (2014).
Krammer, F., Palese, P. & Steel, J. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr. Top. Microbiol. Immunol. 386, 301–321 (2015).
Nabel, G.J. & Fauci, A.S. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat. Med. 16, 1389–1391 (2010).
Altenburg, A.F., Rimmelzwaan, G.F. & de Vries, R.D. Virus-specific T cells as correlate of (cross-)protective immunity against influenza. Vaccine 33, 500–506 (2015).
Sridhar, S. et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 19, 1305–1312 (2013).
Wilkinson, T.M. et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18, 274–280 (2012).
Zens, K.D. & Farber, D.L. Memory CD4 T cells in influenza. Curr. Top. Microbiol. Immunol. 386, 399–421 (2015).
Impagliazzo, A. et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 349, 1301–1306 (2015).
Schwartzman, L.M. et al. An intranasal virus-like particle vaccine broadly protects mice from multiple subtypes of influenza A virus. MBio. 6, e01044 (2015).
Yassine, H.M. et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 21, 1065–1070 (2015).
Rose, M.A., Zielen, S. & Baumann, U. Mucosal immunity and nasal influenza vaccination. Expert Rev. Vaccines 11, 595–607 (2012).
Asahi-Ozaki, Y. et al. Intranasal administration of adjuvant-combined recombinant influenza virus HA vaccine protects mice from the lethal H5N1 virus infection. Microbes Infect. 8, 2706–2714 (2006).
Barria, M.I. et al. Localized mucosal response to intranasal live attenuated influenza vaccine in adults. J. Infect. Dis. 207, 115–124 (2013).
De Filette, M. et al. Universal influenza A vaccine: optimization of M2-based constructs. Virology 337, 149–161 (2005).
Herve, P.L. et al. A novel subnucleocapsid nanoplatform for mucosal vaccination against influenza virus that targets the ectodomain of matrix protein 2. J. Virol. 88, 325–338 (2014).
Price, G.E. et al. Mucosal immunization with a candidate universal influenza vaccine reduces virus transmission in a mouse model. J. Virol. 88, 6019–6030 (2014).
Stary, G. et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348, aaa8205 (2015).
Sui, Z. et al. Cross-protection against influenza virus infection by intranasal administration of M1-based vaccine with chitosan as an adjuvant. Vaccine 28, 7690–7698 (2010).
Teijaro, J.R. et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187, 5510–5514 (2011).
Seibert, C.W. et al. Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs. J. Virol. 87, 7793–7804 (2013).
Friesen, R.H. et al. A common solution to group 2 influenza virus neutralization. Proc. Natl. Acad. Sci. USA 111, 445–450 (2014).
Hamada, H. et al. Multiple redundant effector mechanisms of CD8+ T cells protect against influenza infection. J. Immunol. 190, 296–306 (2013).
Neirynck, S. et al. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 5, 1157–1163 (1999).
Fiers, W. et al. M2e-based universal influenza A vaccine. Vaccine 27, 6280–6283 (2009).
Ito, T. et al. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J. Virol. 65, 5491–5498 (1991).
Liu, W. et al. Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect. 7, 171–177 (2005).
Schotsaert, M. et al. Universal M2 ectodomain-based influenza A vaccines: preclinical and clinical developments. Expert Rev. Vaccines 8, 499–508 (2009).
Simmons, C.P. et al. MHC class I-restricted cytotoxic lymphocyte responses induced by enterotoxin-based mucosal adjuvants. J. Immunol. 163, 6502–6510 (1999).
Turley, C.B. et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine 29, 5145–5152 (2011).
Fiers, W. et al. Soluble recombinant influenza vaccines. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 1961–1963 (2001).
Lamb, R.A., Zebedee, S.L. & Richardson, C.D. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell 40, 627–633 (1985).
DiLillo, D.J. et al. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat. Med. 20, 143–151 (2014).
El Bakkouri, K. et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J. Immunol. 186, 1022–1031 (2011).
Deng, L. et al. M2e-based universal influenza A vaccines. Vaccines (Basel) 3, 105–136 (2015).
Eliasson, D.G. et al. CTA1-M2e-DD: a novel mucosal adjuvant targeted influenza vaccine. Vaccine 26, 1243–1252 (2008).
Eliasson, D.G. et al. A novel non-toxic combined CTA1-DD and ISCOMS adjuvant vector for effective mucosal immunization against influenza virus. Vaccine 29, 3951–3961 (2011).
Agren, L.C. et al. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J. Immunol. 158, 3936–3946 (1997).
Misplon, J.A. et al. Genetic control of immune responses to influenza A matrix 2 protein (M2). Vaccine 28, 5817–5827 (2010).
Mozdzanowska, K. et al. Induction of influenza type A virus-specific resistance by immunization of mice with a synthetic multiple antigenic peptide vaccine that contains ectodomains of matrix protein 2. Vaccine 21, 2616–2626 (2003).
Tompkins, S.M. et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg. Infect. Dis. 13, 426–435 (2007).
Goodman, A.G. et al. A human multi-epitope recombinant vaccinia virus as a universal T cell vaccine candidate against influenza virus. PLoS ONE 6, e25938 (2011).
Droebner, K. et al. Antibodies and CD4(+) T-cells mediate cross-protection against H5N1 influenza virus infection in mice after vaccination with a low pathogenic H5N2 strain. Vaccine 26, 6965–6974 (2008).
Hillaire, M.L., Osterhaus, A.D. & Rimmelzwaan, G.F. Induction of virus-specific cytotoxic T lymphocytes as a basis for the development of broadly protective influenza vaccines. J. Biomed. Biotechnol. 2011, 939860 (2011).
McKinstry, K.K. et al. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J. Clin. Invest. 122, 2847–2856 (2012).
Strutt, T.M. et al. Multipronged CD4(+) T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol. Rev. 255, 149–164 (2013).
Bemark, M. et al. A unique role of the cholera toxin A1-DD adjuvant for long-term plasma and memory B cell development. J. Immunol. 186, 1399–1410 (2011).
Bar-On, L. & Jung, S. Defining in vivo dendritic cell functions using CD11c-DTR transgenic mice. Methods Mol. Biol. 595, 429–442 (2010).
Anderson, K.G. et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 9, 209–222 (2014).
La Gruta, N.L. & Turner, S.J. T cell mediated immunity to influenza: mechanisms of viral control. Trends Immunol. 35, 396–402 (2014).
Hale, J.S. & Ahmed, R. Memory T follicular helper CD4 T cells. Front. Immunol. 6, 16 (2015).
De Baets, S. et al. A GFP expressing influenza A virus to report in vivo tropism and protection by a matrix protein 2 ectodomain-specific monoclonal antibody. PLoS ONE 10, e0121491 (2015).
Wiersma, L.C., Rimmelzwaan, G.F. & de Vries, R.D. Developing universal influenza vaccines: hitting the nail, not just on the head. Vaccines (Basel) 3, 239–262 (2015).
Ye, P. et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194, 519–527 (2001).
Russell, S.M. & Liew, F.Y. T cells primed by influenza virion internal components can cooperate in the antibody response to haemagglutinin. Nature 280, 147–148 (1979).
McKinstry, K.K., Strutt, T.M. & Swain, S.L. Hallmarks of CD4 T cell immunity against influenza. J. Intern. Med. 269, 507–518 (2011).
Scherle, P.A., Palladino, G. & Gerhard, W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J. Immunol. 148, 212–217 (1992).
Sangster, M.Y. et al. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J. Exp. Med. 198, 1011–1021 (2003).
MacLeod, M.K. et al. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol. 186, 2889–2896 (2011).
Epstein, S.L. et al. Vaccination with DNA encoding internal proteins of influenza virus does not require CD8(+) cytotoxic T lymphocytes: either CD4(+) or CD8(+) T cells can promote survival and recovery after challenge. Int. Immunol. 12, 91–101 (2000).
Liang, S. et al. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J. Immunol. 152, 1653–1661 (1994).
Mozdzanowska, K. et al. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J. Virol. 79, 5943–5951 (2005).
Tamura, S. et al. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J. Immunol. 156, 3892–3900 (1996).
Ulmer, J.B. et al. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J. Virol. 72, 5648–5653 (1998).
Valkenburg, S.A. et al. IL-15 adjuvanted multivalent vaccinia-based universal influenza vaccine requires CD4+ T cells for heterosubtypic protection. Proc. Natl. Acad. Sci. USA 111, 5676–5681 (2014).
Brown, D.M. et al. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J. Immunol. 177, 2888–2898 (2006).
Lai, W. et al. Transcriptional control of rapid recall by memory CD4 T cells. J. Immunol. 187, 133–140 (2011).
McKinstry, K.K. et al. Memory CD4 T cell-mediated immunity against influenza A virus: more than a little helpful. Arch. Immunol. Ther. Exp. (Warsz) 61, 341–353 (2013).
Strutt, T.M. et al. Memory CD4+ T-cell-mediated protection depends on secondary effectors that are distinct from and superior to primary effectors. Proc. Natl. Acad. Sci. USA 109, E2551–E2560 (2012).
Wang, C., Collins, M. & Kuchroo, V.K. Effector T cell differentiation: are master regulators of effector T cells still the masters? Curr. Opin. Immunol. 37, 6–10 (2015).
Kudva, A. et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J. Immunol. 186, 1666–1674 (2011).
Gopal, R. et al. Mucosal pre-exposure to Th17-inducing adjuvants exacerbates pathology after influenza infection. Am. J. Pathol. 184, 55–63 (2014).
Maroof, A. et al. Intranasal vaccination promotes detrimental Th17-mediated immunity against influenza infection. PLoS Pathog. 10, e1003875 (2014).
Moser, E.K. et al. IL-21R signaling suppresses IL-17+ gamma delta T cell responses and production of IL-17 related cytokines in the lung at steady state and after influenza A virus infection. PLoS ONE 10, e0120169 (2015).
Li, W., Moltedo, B. & Moran, T.M. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J. Virol. 86, 12304–12312 (2012).
Lim, K. et al. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science 349, aaa4352 (2015).
Sell, S. et al. Intraepithelial T-cell cytotoxicity, induced bronchus-associated lymphoid tissue, and proliferation of pneumocytes in experimental mouse models of influenza. Viral Immunol. 27, 484–496 (2014).
Moyron-Quiroz, J.E. et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10, 927–934 (2004).
Schotsaert, M. et al. Natural and long-lasting cellular immune responses against influenza in the M2e-immune host. Mucosal Immunol. 6, 276–287 (2013).
Christensen, D. et al. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol. 10, 260–270 (2016).
Zens, K.D., Chen, J.K. & Farber, D.L. Vaccine-generated lung tissue–resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 1, e85832 (2016).
Cho, K.J. et al. Crystal structure of the conserved amino-terminus of the extracellular domain of matrix protein 2 of influenza A virus gripped by an antibody. J. Virol. 90, 611–615 (2015).
Cho, K.J. et al. Structure of the extracellular domain of matrix protein 2 of influenza A virus in complex with a protective monoclonal antibody. J. Virol. 89, 3700–3711 (2015).
Leung, H.C. et al. An H5N1-based matrix protein 2 ectodomain tetrameric peptide vaccine provides cross-protection against lethal infection with H7N9 influenza virus. Emerg. Microbes Infect. 4, e22 (2015).
Scorza, F.B., Tsyetnitsky, V. & Donnelly, J.J. Universal influenza vaccines: shifting to better vaccines. Vaccine 34, 2926–2933 (2016).
Eriksson, A.M., Schon, K.M. & Lycke, N.Y. The cholera toxin-derived CTA1-DD vaccine adjuvant administered intranasally does not cause inflammation or accumulate in the nervous tissues. J. Immunol. 173, 3310–3319 (2004).
Mutsch, M. et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350, 896–903 (2004).
Stephenson, I. et al. Phase I evaluation of intranasal trivalent inactivated influenza vaccine with nontoxigenic Escherichia coli enterotoxin and novel biovector as mucosal adjuvants, using adult volunteers. J. Virol. 80, 4962–4970 (2006).
White, J. et al. Two better cell lines for making hybridomas expressing specific T cell receptors. J. Immunol. 143, 1822–1825 (1989).
Acknowledgements
A special thanks to the NIH Tetramer Core Facility for producing the unique MHC class II-restricted M2e tetramer: I-A(d) Influenza A M2 2-17 SLLTEVETPIRNEWGS varC>S. This study was supported by research funds from the Swedish Research Council, the Swedish Cancer Foundation, Swedish Foundation for Strategic Research, Knut och Alice Wallenbergs Stiftelse, EU projects in FP7-PEOPLE-2013-ITN UniVacFlu, and FP7-HEALTH-2013-INNOVATION-1 UNISEC. We also thank staff at the Centre for Cellular Imaging and the Laboratory for Experimental Biomedicine at the University of Gothenburg for valuable help with the studies. Research on M2e in the group of Saelens was supported by FP7-ITN project UNIVACFLU, FWO-project G052412N, and Belgian Federal Sciences Administration Project BELVIR p7/45. L.D. was supported by State Scholarship Fund (File No. 2011674067) from the China Scholarship Council and by IUAP-BELVIR p7/45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Eliasson, D., Omokanye, A., Schön, K. et al. M2e-tetramer-specific memory CD4 T cells are broadly protective against influenza infection. Mucosal Immunol 11, 273–289 (2018). https://doi.org/10.1038/mi.2017.14
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2017.14
This article is cited by
-
Double-layered N-S1 protein nanoparticle immunization elicits robust cellular immune and broad antibody responses against SARS-CoV-2
Journal of Nanobiotechnology (2024)
-
Validation of Multi-epitope Peptides Encapsulated in PLGA Nanoparticles Against Influenza A Virus
Pharmaceutical Research (2023)
-
ADP-ribosylating adjuvant reveals plasticity in cDC1 cells that drive mucosal Th17 cell development and protection against influenza virus infection
Mucosal Immunology (2022)
-
Clonotypic analysis of protective influenza M2e-specific lung resident Th17 memory cells reveals extensive functional diversity
Mucosal Immunology (2022)
-
A vaccine combination of lipid nanoparticles and a cholera toxin adjuvant derivative greatly improves lung protection against influenza virus infection
Mucosal Immunology (2021)