Abstract
The complement subunit C1q was recently identified as a marker for monocyte-derived regulatory dendritic cells supporting the differentiation of interleukin (IL)-10-secreting CD4+ T cells with a suppressive activity. Furthermore, C1q expression is upregulated in peripheral blood mononuclear cells of allergic patients in the course of successful allergen immunotherapy. Herein, we investigated a potential direct role of C1q in downregulating allergic inflammation. In mice with ovalbumin (OVA) or birch pollen (BP)-induced allergic asthma, C1q is as efficacious as dexamethasone to reduce both airway hyperresponsiveness (AHR), eosinophil, and ILC2 infiltrates in bronchoalveolar lavages, as well as allergen-specific T helper 2 cells in the lungs. Administration of C1q does not expand IL-10+/Foxp3+ regulatory T cells in the lungs, spleen, or in the blood. Depletion of plasmacytoid dendritic cells (pDCs) abrogates the capacity of C1q to reduce AHR and eosinophilic infiltrates in OVA-sensitized mice. Also C1q treatment inhibits the activation of human and mouse pDCs by CpGs, thereby demonstrating a critical role for pDCs in the anti-inflammatory activity of C1q. We conclude that regulatory dendritic cells can mediate a potent direct anti-inflammatory activity via the expression and/or secretion of molecules such as C1q, independently of their capacity to expand the pool of regulatory T cells.
Similar content being viewed by others
Introduction
The C1q molecule, present in serum, is a well-known component of the classical complement cascade. It is also expressed at the surface or secreted by antigen-presenting cells (APCs) such as monocytes, macrophages, and monocyte-derived dendritic cells (MoDCs).1, 2 Recently, we identified C1q as a regulatory dendritic cell (DCreg) marker based on the observation that dexamethasone (DEX)-, interleukin (IL)-10- or vitamin D3-treated MoDCs, all capable to induce the differentiation of naive CD4+ T cells into regulatory T (Treg) cells, upregulate C1q by at least 10-fold at both mRNA and protein levels.3 We also observed an increased expression of the C1q genes encoding each of its A, B and C subunits, in peripheral blood mononuclear cells (PBMCs) from grass pollen allergic patients in relationship with clinical benefit in the course of allergen immunotherapy.3 Consistent with these results, it has been proposed that C1q at the surface of human macrophages or dendritic cells (DCs) acts as a scavenger receptor involved in the clearance of apoptotic bodies, thus reducing pro-inflammatory immune responses.4, 5, 6 Also, C1q deficiency in human is associated with severe autoimmune diseases, including systemic lupus erythematosus as well as glomerulonephritis.7, 8, 9, 10 Whereas these observations argue for a functional role of surface-expressed and/or secreted C1q in DCregs, a potential direct effect of this molecule in downregulating inflammation has never been reported.
In this context, we investigated herein the biological activity of soluble C1q in murine models of T helper 2 (Th2)-driven lung inflammation. We demonstrate that C1q in itself is a potent inhibitor of allergic asthma and further, that it acts directly via plasmacytoid DCs (pDCs), in the absence of any expansion of Treg cells.
Results
C1q exhibits an anti-inflammatory activity in murine asthma models
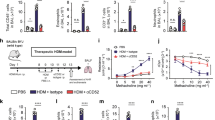
The therapeutic effect of the human complement C1q subunit was tested in vivo in a murine model of induced asthma to ovalbumin (OVA) (Figure 1a). OVA-sensitized mice treated intraperitoneally (IP) with phosphate-buffered saline (PBS) displayed a high airway hyperresponsiveness (AHR) to methacholine, measured by whole-body plethysmography as a Penh index, in comparison with healthy (i.e., non-sensitized) animals (Figure 1b). IP administration of purified C1q induced a significant (P<0.01, 50 μg per dose) reduction of AHR when compared with PBS-treated mice (Figure 1b). A statistically significant decrease in both eosinophil counts in bronchoalveolar lavages (BALs) and Th2 cytokine (i.e., IL-5 and IL-13) secretion by in vitro re-stimulated OVA-specific T cells recovered from the lungs was also observed in animals receiving doses of 50 or 100 μg of C1q (Figure 1c–e).
C1q treatment significantly reduces airway inflammation in ovalbumin (OVA)-sensitized mice. (a) Study design. (b) Airway hyperresponsiveness (AHR) was determined by measuring the Penh index in presence of methacholine (50 mg ml−1). (c) Percentages of eosinophils were determined in bronchoalveolar lavages by using flow cytometry. (d, e) Levels of interleukin (IL)-5 and IL-13 were assessed in culture supernatants of OVA-stimulated lung cells by using a multiplex cytokine quantification assay. Horizontal bars represent the mean responses±s.e.m., with n=5–7 mice per group. Data were compared using the nonparametric Kruskal–Wallis test. NS, non-significant, *P<0.05, and **P<0.01. IP, intraperitoneal; PBS, phosphate-buffered saline.
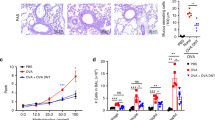
In a second set of experiments, C1q was thermodenatured at 84 °C for 10 min to alter irreversibly its secondary structure, as confirmed by circular dichroism (CD) analyses (Supplementary Figure S1 online). Heat-denatured C1q was then tested in the aforementioned asthma model in comparison with DEX used as a positive control (Figure 2a). OVA-sensitized mice treated IP with either C1q or DEX exhibited a comparable decrease in both AHR (P<0.01) and pulmonary resistance (P<0.05 and P<0.01, respectively) when compared with the PBS group (Figure 2b,c). These changes observed in C1q- or DEX-treated mice were associated with a significant reduction in eosinophils (P<0.05 and P<0.01, respectively) and ILC2s (P<0.05) in BALs (Figure 2d,e). In contrast, administration of heat-denatured C1q had no effect on AHR, pulmonary resistance nor on inflammatory cell infiltrates in BALs (Figure 2b–e). A significant decrease in IL-5 and IL-13 secretion by in vitro re-stimulated OVA-specific T cells recovered from the lungs was observed in both C1q- (P<0.01 and P<0.05, respectively) and DEX- (P<0.001 and P<0.01, respectively) treated mice, but not in mice receiving heat-denatured C1q (Figure 2f,g). Although not shown, none of those treatments had any effect on OVA-specific serum IgE titers.
Treatment with heat-denatured C1q has no impact on airway inflammation in ovalbumin (OVA)-sensitized mice. (a) Study design. (b, c) Airway hyperresponsiveness (AHR) was determined by measuring the Penh index or bronchial resistance in response to methacholine (using 50 mg ml−1 and a dose range from 1.87 to 15 mg ml−1, respectively). (d, e) Percentages of eosinophils and ILC2s were determined in bronchoalveolar lavages by using flow cytometry. (f, g) Levels of interleukin (IL)-5 and IL-13 were assessed in culture supernatants of OVA-stimulated lung cells by using a multiplex cytokine quantification assay. Horizontal bars represent the mean responses±s.e.m., with n=6 mice per group. Data were compared using the nonparametric Kruskal–Wallis test. NS, non-significant, *P<0.05, **P<0.01, and ***P<0.001. DEX, dexamethasone; IP, intraperitoneal; PBS, phosphate-buffered saline.
We subsequently proceeded to confirm the anti-inflammatory activity of C1q in asthmatic mice sensitized to a natural allergen extract obtained from BP (Figure 3a). Mice exposed to BP exhibited a strong AHR, as well as eosinophil infiltrates in BALs associated with IgE and Th2 responses against the major BP allergen Bet v 1.11 Both C1q and DEX treatment reduced these readouts (with most of these reductions reaching statistical significance) when compared with the PBS group (Figure 3b–e), thus confirming the anti-inflammatory effect of C1q in animals sensitized to a natural allergen source.
C1q treatment significantly reduces airway inflammation in birch pollen allergic mice. (a) Study design. (b) Airway hyperresponsiveness (AHR) was determined by measuring the Penh index in response to methacholine (50 mg ml−1). (c) Percentages of eosinophils were determined in bronchoalveolar lavages by using flow cytometry. (d, e) Levels of interleukin (IL)-5 and IL-13 were assessed in culture supernatants of birch pollen-stimulated lung cells by using a multiplex cytokine quantification assay. Horizontal bars represent the mean responses±s.e.m., with n=4–6 mice per group. Data were compared using the nonparametric Kruskal–Wallis test. NS, non-significant, *P<0.05, and **P<0.01. DEX, dexamethasone; IP, intraperitoneal; PBS, phosphate-buffered saline.
C1q treatment does not promote the expansion of Treg cells
C1q expression or secretion being a marker of regulatory DCs,3 we then assessed whether administration of soluble C1q resulted in Treg cell expansion. Following treatment with C1q as per the protocol described in Figure 1a, no increase of CD4+ CD25+ Foxp3+ T cells was observed either in the lungs or in the spleen of OVA-sensitized mice (Figure 4a). Although not shown, the expression of regulatory markers such as Helios, neuropilin 1, KLRG1, and CD103 was also unmodified in CD4+ CD25+ Foxp3+ Tregs from these mice. In contrast, IL-10 production was rather decreased in OVA-stimulated T cells recovered from the lungs or spleen of C1q-treated mice, when compared with the PBS group (Figure 4b; P<0.05), likely as a decrease in Th2 responses.
C1q treatment does not induce regulatory T (Treg) cells. (a) Percentages of Foxp3+ cells were assessed among CD4+ CD25+ T cells in the lungs or spleens of ovalbumin (OVA)-sensitized mice treated intraperitoneally (IP) with either phosphate-buffered saline (PBS) or C1q (50 μg per dose for 4 consecutive days before aerosol challenges), by using flow cytometry. (b) Levels of interleukin (IL)-10 were assessed in culture supernatants of OVA-stimulated lung cells from OVA-sensitized mice treated IP with either PBS or C1q (50 μg per dose for 4 consecutive days before aerosol challenges), by using a multiplex cytokine quantification assay. (c, d) Percentages of regulatory (CD4+ CD44+ Foxp3+) and effector (CD4+ CD44+ Foxp3−) T cells were assessed in the blood of naive mice treated IP with either PBS, C1q (50 μg), or IL-2 (25,000 IU) for 5 consecutive days. Horizontal bars represent the mean responses±s.e.m., with n=6 mice per group. Data were compared using the nonparametric Kruskal–Wallis test. NS, non-significant, *P<0.05, and **P<0.01.
To confirm the previous results, we analyzed the impact of C1q administration to naive mice in comparison with a low-dose (25,000 IU) regimen of IL-2 known to induce functional CD4+ CD44+ Foxp3+ Tregs.12 After 5 consecutive days of IP treatment with low dose IL-2, a significant upregulation of CD4+ CD44+ Foxp3+ Tregs, concomitantly with a decrease of CD4+ CD44+ Foxp3− effector T cells (T effs) was observed in the blood of naive mice (Figure 4c,d). In contrast, parallel treatment with 50 μg of C1q per dose failed to induce such Foxp3+ Tregs (Figure 4c,d). Although not shown, we also failed following treatment with C1q to detect any expansion of Foxp3+ Tregs in the mesenteric lymph nodes of naive mice adoptively transferred with CD4+ Thy1.1+GFP− T cells from Foxp3gfp mice (i.e., animals expressing the GFP gene under the control of the Foxp3 gene promoter). Taken together, we conclude that treatment with soluble C1q does not induce Tregs in either allergen-sensitized or naive mice.
C1q mediates its anti-inflammatory activity through a modulation of pDC function
To identify potential cellular targets of C1q, we analyzed by flow cytometry the expression of known C1q receptors, including cC1qR, gC1qR, CD93, CR1 (CD35), and LAIR-1 in human blood cells (Figure 5). Interestingly, several DC subsets including MoDCs, mDCs as well as pDCs, respectively, but not monocytes, T and natural killer (NK) cells, express cC1qR. Similarly, gC1qR and CD93 are mainly detected in most DC subsets and monocytes but not in other leukocytes (i.e., B, T and NK cells). LAIR-1 is detected in mDCs, pDCs, monocytes, and cells of the lymphocytic lineage, whereas mDCs, monocytes, and B cells express CR1 at their surface.
Surface expression of C1q receptors in human leukocyte subsets. Monocytes, monocyte-derived dendritic cells (MoDCs), myeloid dendritic cells (mDCs), plasmacytoid dendritic cells (pDCs), B, T, and natural killer (NK) cells were analyzed for the expression of cC1q, gC1q, CD93, CR1, and LAIR-1 by flow cytometry using specific antibodies. Results are expressed as mean percentages of positive cells±s.e.m., analyzed in blood samples from three healthy donors.
On the basis of these results, we investigated a potential functional impact of C1q on various DC subsets. In a first set of experiments, we failed to detect any inhibitory effect of C1q on the activation of MoDCs and mDCs stimulated by lipopolysaccharide+interferon (IFN)γ or poly I:C, respectively, under in vitro serum-free conditions ( Supplementary Figure S2 ). If anything, C1q rather increased IL-12p70 and tumor necrosis factor (TNF)-α secretion by stimulated mDCs ( Supplementary Figure S2 ). Interestingly, however, pDCs incubated with C1q and stimulated by CpGA secreted significantly (P<0.01) less pro-inflammatory cytokines (including IFN-α, IL-8, and TNF-α) when compared with pDCs stimulated by CpGA alone (Figure 6a). As expected, non-treated or unstimulated C1q-treated pDCs secreted only background levels of such pro-inflammatory cytokines (Figure 6a). Consistent with these results, murine pDCs stimulated with CpGA in presence of C1q secreted lower levels of cytokines when compared with CpGA-activated pDCs (Figure 6b). In contrast, C1q had no impact on the production of cytokines (i.e., IL-6 and TNF-α) by either bone-marrow-derived DCs or conventional DCs stimulated by lipopolysaccharide ( Supplementary Figure S3 ). In addition, although not shown, co-culture experiments with treated pDCs and allogeneic naive CD4+ T cells confirmed that such a downregulation of the production of pro-inflammatory cytokines in pDCs results in a reduced secretion of IFNγ, IL-4, and IL-13 by co-cultured CD4+ T cells.
Plasmacytoid dendritic cells (pDCs) are critical for the anti-inflammatory activity of C1q. Cytokine production by (a) human or (b) murine pDCs treated with C1q (10 μg ml−1), CpGA (2 μg ml−1), or a combination of CpGA and C1q was analyzed after 24 h by using a multiplex cytokine quantification assay. Data are shown as means±s.e.m. (n=6) and were compared using the nonparametric Wilcoxon test. NS, non-significant, *P<0.05, and **P<0.01. (c) Ovalbumin (OVA)-sensitized mice were treated intraperitoneally (IP) 1 h before each OVA aerosol challenge with either dexamethasone (60 μg per dose) or native C1q (50 μg per dose). In some groups, mice were treated IP with either 50 μg of the 120G8 mAb to deplete pDCs, or a monoclonal antibody (mAb) with a corresponding isotype as a control, 2 h before each OVA challenge for 4 consecutive days. Airway hyperresponsiveness (AHR) measurements in response to methacholine (50 mg ml−1) and percentages of eosinophils in bronchoalveolar lavages were assessed at days 25 and 26, respectively. Horizontal bars represent the mean responses±s.e.m., with n=10 mice per group. Data were compared using the nonparametric Kruskal–Wallis test. NS, non-significant and *P<0.05. IFN-α, interferon-α; IL-8, interleukin-8; NT, non-treated; TNF-α, tumor necrosis factor-α.
To further investigate whether the inhibitory activity of C1q on allergic inflammation occurs through a modulation of pDC function, we depleted pDCs in OVA-sensitized mice following injection of the 120G8 monoclonal antibody (mAb), 2 h before each individual OVA challenge. The depletion of pDCs in those mice was virtually complete, as confirmed by fluorescence-activated cell sorting analysis (data not shown). pDC-depleted mice sensitized to OVA exhibited a high AHR as well as eosinophil infiltrates in their BALs (Figure 6c) comparable to those of OVA-sensitized animals treated with a control isotype mAb, establishing that the loss of pDCs per se does not alter sensitization. Interestingly, the inhibitory effect of C1q treatment on both AHR and eosinophil counts in BALs was totally abrogated in animals with pDC depletion (Figure 6c), with levels for those readouts similar to those observed in OVA-sensitized mice. Collectively, these results confirm that C1q acts via pDCs to inhibit allergic inflammation in this murine model.
Discussion
C1q is a serum protein involved in the activation of the complement cascade. C1q is also expressed at the surface and/or secreted by monocytes and monocyte-derived macrophages and DCs.1, 2 Several studies have documented a role for this molecule in the clearance of apoptotic cells through the formation of C1q/apoptotic body complexes, thereby facilitating the rapid elimination of dying cells and preventing the release of damaging intracellular components.4, 5, 6, 9 We recently identified C1q as a bona fide regulatory DC marker, as the molecule is overexpressed in MoDCs secreting IL-10 and supporting the differentiation of Treg cells.3 We also demonstrated that during allergen immunotherapy, the upregulation of C1q in PBMCs of allergic patients is correlated with clinical benefit.3 Genetic deficiency in C1q in humans is associated with conditions reflecting a loss of tolerance, including for example, severe systemic lupus erythematosus.7, 8, 9, 10 Whereas all these observations are consistent with the hypothesis that cellular expression and/or secretion of C1q is a marker of regulatory immune responses, as of today however, the potential role of C1q in the tolerogenic function of DCregs remains unclear.
In this context, we undertook in the present study to assess the anti-inflammatory activity of C1q in OVA- or BP-sensitized BALB/c mice, as models of Th2-driven allergic asthma. Administration to such animals of soluble C1q, but not heat-denatured C1q, decreased AHR, reduced eosinophil and ILC2 infiltrates in BALs, and downregulated Th2 responses in the lungs to levels similar to those observed when administering DEX. Although not shown, in a murine model of experimental autoimmune encephalomyelitis, treatment with C1q did not prevent encephalomyelitis development, but nonetheless clearly delayed the onset of the disease, suggesting a capacity of the molecule to inhibit as well Th1/Th17-mediated inflammation.
Having documented a significant anti-inflammatory activity of C1q, we subsequently investigated the mechanisms involved. Importantly, C1q mediates its anti-inflammatory activity independently of any expansion of IL-10+/Foxp3+ Tregs, as demonstrated either in OVA-sensitized or in naive mice receiving C1q. The latter observation illustrates that regulatory DCs contribute to tolerance induction not only by supporting the differentiation of naive CD4+ T cells into IL-10-producing Tregs with a suppressive capacity but also through the expression and/or secretion of molecules such as C1q with a direct anti-inflammatory activity. Fluorescence-activated cell sorting analysis of surface receptors for C1q in human leukocytes led to the hypothesis that C1q could directly act on DCs, including MoDCs, mDCs, and pDCs. We did not observe, however, any inhibitory effect of either immobilized or soluble C1q on the activation of MoDCs or mDCs ( Supplementary Figure S2 and data not shown). The latter is in contrast with previous evidence that immobilized C1q can inhibit the production of pro-inflammatory cytokines (such as IL1-β, IL-6, IL-8, IL-12, IL-23, and TNF-α) by MoDCs, thereby downregulating Th1/Th17 responses while inducing Tregs.4, 13 The reason for this discrepancy is unclear, but could possibly be explained by different culture conditions used in those various experiments. Interestingly, in our hands, treatment with soluble (Figure 6a) or immobilized (data not shown) C1q downregulates the production of pro-inflammatory cytokines (such as IFN-α, IL-8, and TNF-α) by pDCs when stimulated with CpGA. Our results are fully consistent with a previous report documenting an increased production of IFN-α by pDCs in C1q-deficient patients.14 In our murine asthma model, a selective depletion of pDCs abrogated the capacity of C1q treatment to downregulate allergic inflammation. This observation is in line with previous data suggesting a role for pDCs in decreasing allergic inflammation. For example, the recruitment of pDCs in the lungs markedly suppresses airway inflammation in mice.15 Also, numbers of circulating pDCs are significantly lower in children with allergic asthma, when compared with healthy children.16, 17 Nonetheless, the precise mechanism by which C1q treatment potentiates an anti-inflammatory activity mediated via pDCs remains to be addressed. Specifically, the receptor(s) involved in the inhibitory activity of C1q on pDCs remain(s) to be characterized. LAIR-1 is a possible candidate as observed by others in human APCs,18, 19 even if we failed to detect any impact of siRNA downregulation of this receptor on C1q function in pDCs ( Supplementary Figures S4 and S5 ). Besides their capacity to induce Treg cells,20, 21, 22 which seems in our model unlikely, pDCs could inhibit allergic inflammation either by (i) competing with mDCs during allergen uptake,23 (ii) inhibiting directly mDC functions,15, 24 or (iii) decreasing proliferation and/or cytokine production by Th2 cells.25 From this, we hypothesize that modulation of pDC function by C1q induced in blood PBMCs during allergen immunotherapy contributes to clinical improvement in allergic patients.3
Altogether, our study confirms the relevance of C1q as a marker for DCregs, as this molecule exhibits per se an anti-inflammatory activity in Th2-driven asthma models. From these results, we infer that DCregs can mediate a direct anti-inflammatory activity, independently of the induction of Treg cells, which contributes to allergen immunotherapy efficacy.
Methods
Mice and reagents. Six- to eight-week-old BALB/c female mice were obtained from Charles River (L’Arbresle, France). The experimental protocol (no. 01684.02) was approved by Stallergenes Greer (Antony, France) ethical committee (COREA No. 117). Animal handling was performed according to the international regulations. PBS, Dulbecco’s PBS, and Roswell Park Memorial Institute (RPMI) 1640 were purchased from Life Technology (Saint-Aubin, France). For mouse in vitro experiments, the culture medium used consisted of RPMI 1640 supplemented with 10% fetal calf serum, 1% L-glutamine, 200 U ml−1 penicillin, and 200 μg ml−1 streptomycin (all from Life Technology). For human in vitro experiments, the CellGro GMP serum-free DC medium (CellGenix, Freiburg, Germany) supplemented with 10 μg ml−1 gentamicin (Life Technology) was used. OVA grade V with low endotoxin content was purchased from Sigma (St Louis, MO) and was further purified on an endotoxin-removing gel (Pierce, Rockford, IL). Residual endotoxin concentrations determined by the Endochrome-K assay (R1708K, Charles River, Wilmington, MA) were always <0.1 EU per μg protein. Human purified C1q was obtained from Calbiochem, Merck Millipore (Darmstadt, Germany) and DEX was purchased from Sigma. Aqueous BP extracts were produced by Stallergenes Greer.
For analysis of inflammatory cells in BALs, the following mAbs were used and labeled with either fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), or PE-APC: anti-Gr1 (RB6-8C5; BD Biosciences, Le Pont de Claix, France); anti-B220 (RA3-6B2); anti-CD3 (145-2C11); anti-CD4 (GK1.5); anti-CD8 (53-6.7); anti-CD11b (M1/70); anti-CD11c (N418); anti-CD19 (1D3); anti-FcɛRI (MAR-1), and anti-ICOS (C398.4A) (all from eBiosciences, San Diego, CA); anti-T1/ST2 (DJ8; MD Bioscience, St Paul, MN); and anti-CCR3 (83101; R&D Systems, Lille, France). Corresponding isotype-matched mAbs were used as controls.
Treatment with C1q of BALB/c mice with OVA- or BP-induced asthma. Mice were sensitized following IP injection on days 0 and 14 of either OVA (10 μg) or BP extracts (using doses equivalent to 10 μg Bet v 1) adsorbed on 2 mg Al(OH)3 (Pierce), administered in 100 μl PBS. At each of days 21–24, a 20 min aerosol challenge was performed with 1% w/v OVA or BP extracts (equivalent to 1 mg Bet v 1) using an aerosol delivery system (Buxco Europe, Winchester, UK). C1q (10–100 μg per dose) or thermodenatured C1q (50 μg per dose) were administered IP 1 h before each aerosol challenge. PBS or DEX (60 μg per dose) were administered IP as negative and positive controls, respectively. In selected experiments, mice were treated IP with 50 μg of the 120G8 mAb (Dendritics, Lyon, France) for 4 consecutive days, starting 2 h before each OVA challenge in order to deplete pDCs, as confirmed by flow cytometry analysis of splenocytes.
Measurements of AHR were performed by whole-body plethysmography (Buxco) after exposure to increasing doses (i.e., 6.125, 12.5, 25, and 50 mg ml−1) of methacholine. Results were expressed as enhanced pause (Penh) index as previously described.23 Invasive determination of lung resistance was performed in mice anesthetized with ketamine/xylazine (100 and 10 mg kg−1, respectively, Centravet, Maisons-Alfort, France) administered IP after a careful orotracheal intubation. Bronchial resistance was measured using the FinePointe RC system (Buxco) after exposure to increasing doses (i.e., 1.875, 3.75, 7.5, and 15 mg ml−1) of methacholine using a protocol adapted from Swedin and colleagues.26
For analysis of inflammatory cells in BALs, mice were anesthetized with pentobarbital/xylazine (50 and 10 mg kg−1, respectively, Centravet, Maisons-Alfort, France) administered IP and BALs were performed with 3 × 400 μl PBS. BAL fluids were centrifuged at 800g for 10 min at 4 °C. Cells were re-suspended in PBS and stained at 4 °C for 15 min with either anti-CCR3 PE, anti-Gr1 APC, anti-CD3 FITC, and anti-B220 FITC mAbs to assess percentages of eosinophils in BALs, or anti-CD4, anti-CD3, anti-CD8, anti-CD11b, anti-CD11c, anti-CD19, anti-FcɛRI mAbs, all conjugated to PE, as well as anti-T1/ST2 FITC and anti-ICOS APC mAbs to determine percentages of ILC2s in BALs. Corresponding isotype-matched mAbs were used as controls. Samples were acquired by using a FACSVerse apparatus (BD Biosciences) and analyzed with the FlowJo software (FlowJo, Ashland, OR). Percentages of eosinophils were measured as the proportion of CCR3+ CD3− B220− cells among other BAL immune cells, including neutrophils (defined as Gr-1high CD3− CD19− cells), lymphocytes (CD3+ CD19+ cells), and alveolar macrophages (large autofluorescent cells). Percentages of ILC2s were obtained as side scatter (SSC) low, lineage negative (Lin−: CD4− CD3− CD8− CD11b− CD11c− CD19− FcɛRI−) cells expressing ICOS and T1/ST2 (SSClow Lin− ICOS+ T1/ST2+).27
To assess T-cell responses, one lobe from the lungs was incubated for 1 h in RPMI 1640 supplemented with 75 U ml−1 collagenase (Roche, Basel, Switzerland). After blocking residual enzymatic activity with 5 mM EDTA in PBS, lung tissues were dissociated in PBS. Isolated cells were filtered through a 70 μm sieve and washed twice before re-suspension in culture medium. Lung cells were cultured at 106 cells per well in a 96-well plate and incubated with either OVA (100 μg ml−1), a BP extract equivalent to 10 μg ml−1 Bet v 1, or medium alone. After 72 h at 37 °C in 5% CO2, IL-5, IL-10, and IL-13 cytokines were measured in culture supernatants using a mouse cytokine bead kit and a Magpix system (Merck Millipore, Darmstadt, Germany) according to the manufacturer’s instructions.
The analysis of Foxp3 expression was performed in lung and spleen T cells after staining with anti-CD4 PE (GK1.5, eBiosciences) and anti-CD25 FITC (PC61, BD Biosciences) mAbs. After 15 min, cells were fixed, permeabilized, and stained intracellularly with an anti-Foxp3 APC mAb using the Foxp3 staining kit (eBiosciences) according to the manufacturer’s instructions.
Analysis of regulatory Foxp3+ T cells following IL-2 or C1q treatment in naive BALB/c mice. BALB/c mice were treated daily with IP injections of either PBS, 25,000 IU recombinant human IL-2 (Proleukin, Novartis, Rueil-Malmaison, France) or 50 μg of human C1q for 5 consecutive days. Blood was collected at days 0, 5, 8, and 12 and PBMCs were stained for Foxp3 Treg analysis as previously described.12
Analysis of C1q receptors in human leukocytes. The expression of C1q receptors, i.e., cC1qR, gC1qR, and LAIR-1 was analyzed on different subsets of human leukocytes obtained from three healthy donors, including monocytes (CD14+), MoDCs (CD1a+ CD11c+), mDCs (CD11c+ CD1c+ or CD11c+ CD141+), pDCs (CD123+ CD303+), B (CD19+), T (CD3+), and NK cells (CD3− CD56+). Human PBMCs were incubated with the following antibodies specific for either CD1a (BL6; PE; Beckmann Coulter, Villepinte, France), CD1c (L161; APC-Cy7; Biolegend, London, UK), CD3 (UCHT1; PE-Cy7; Beckmann Coulter), CD11c (3.9; APC; Biolegend), CD14 (M5E2; PE; BD Biosciences), CD19 (HIB19; APC; BD Biosciences), CD56 (AF12-7H3; APC Miltenyi Biotech, Paris, France), CD123 (AC145; APC; Miltenyi Biotech), CD141 (AD5-14H12; PE; Miltenyi Biotech), CD303 (AC144; PE; Miltenyi Biotech), cC1qR (R139; FITC; BD Biosciences), gC1qR (60.11; FITC; Abcam, Cambridge, UK), CD93 (R139; FITC; BD Biosciences), CRI (E11; FITC; eBiosciences), or LAIR-1 (NKTA255; FITC; Abcam) for 20 min at 4 °C. Cells were stained in parallel with corresponding isotype-matched mAbs as controls. Samples were acquired by using a FACSVerse apparatus (BD Biosciences) and analyzed with a FlowJo software. Results are expressed as mean percentages of positive cells±s.e.m. obtained from the analysis of blood samples from three healthy donors.
Purification and in vitro stimulation of human pDCs. Human PBMCs were isolated from fresh buffy coats obtained from healthy donors at “Etablissement Français du Sang” (Rungis, France) by centrifugation over a lymphocyte separation medium (Eurobio AbCys, Courtaboeuf, France). pDCs were isolated from PBMCs by negative selection using the MACS pDC isolation kit (Miltenyi Biotec) and an autoMACS Pro Separator (Miltenyi Biotec), according to the manufacturer’s instructions. Such pDCs were confirmed to be >95% pure based on CD123 and CD303 expression evaluated by flow cytometry. pDCs were cultured at 1.105 cells per well in a 96-well plate and incubated with either 2 μg ml−1 CpGA (InvivoGen, Toulouse, France) or serum-free medium alone in presence or absence of 10 μg ml−1 C1q for 24 h at 37 °C and 5% CO2.
Cytokine measurement was performed in supernatants collected 24 h after pDCs treatment using multiplex cytokine quantification assays. IL-8 and TNF-α cytokines were measured using a human cytokine bead kit and a Magpix system (Merck Millipore) according to the manufacturer’s instructions. IFN-α concentrations were measured by ELISA (Tebu-bio, le Perray en Yvelines, France).
Statistical analyses. Results are expressed as means±s.e.m. Statistical differences were assessed by using either the non-parametric Kruskal–Wallis test with subsequent Dunnett’s multiple analyses to compare C1q-, denatured C1q-, DEX-, or IL-2-treated mice with PBS-treated mice, or the non-parametric Wilcoxon test to analyze paired data from human in vitro experiments. P values <0.05 were considered as significant: *P<0.05; **P<0.01; and ***P<0.001. Statistical and graphical analyses were performed using the Prism 6 software (GraphPad, La Jolla, CA).
References
Castellano, G. et al. Immune modulation of human dendritic cells by complement. Eur. J. Immunol. 37, 2803–2811 (2007).
Lu, J.H. et al. The classical and regulatory functions of C1q in immunity and autoimmunity. Cell. Mol. Immunol. 5, 9–21 (2008).
Zimmer, A. et al. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. J. Allergy Clin. Immunol. 129, 1020–1030 (2012).
Fraser, D.A., Laust, A.K., Nelson, E.L. & Tenner, A.J. C1q differentially modulates phagocytosis and cytokine responses during ingestion of apoptotic cells by human monocytes, macrophages, and dendritic cells. J. Immunol. 183, 6175–6185 (2009).
Benoit, M.E., Clarke, E.V., Morgado, P., Fraser, D.A. & Tenner, A.J. Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J. Immunol. 188, 5682–5693 (2012).
Clarke, E.V., Weist, B.M., Walsh, C.M. & Tenner, A.J. Complement protein C1q bound to apoptotic cells suppresses human macrophage and dendritic cell-mediated Th17 and Th1 T cell subset proliferation. J. Leukoc. Biol. 97, 147–160 (2015).
Botto, M. et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19, 56–59 (1998).
Mitchell, D.A. et al. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J. Immunol. 168, 2538–2543 (2002).
Nauta, A.J. et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur. J. Immunol. 32, 1726–1736 (2002).
Orbai, A.M. et al. Anti-C1q antibodies in systemic lupus erythematosus. Lupus 24, 42–49 (2015).
Tourdot, S. et al. Evaluation of therapeutic sublingual vaccines in a murine model of chronic house dust mite allergic airway inflammation. Clin. Exp. Allergy 41, 1784–1792 (2011).
Grinberg-Bleyer, Y. et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J. Exp. Med. 207, 1871–1878 (2010).
Teh, B.K., Yeo, J.G., Chern, L.M. & Lu, J. C1q regulation of dendritic cell development from monocytes with distinct cytokine production and T cell stimulation. Mol. Immunol. 48, 1128–1138 (2011).
Santer, D.M. et al. C1q deficiency leads to the defective suppression of IFN-alpha in response to nucleoprotein containing immune complexes. J. Immunol. 185, 4738–4749 (2010).
Kool, M. et al. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J. Immunol. 183, 1074–1082 (2009).
Hagendorens, M.M. et al. Differences in circulating dendritic cell subtypes in cord blood and peripheral blood of healthy and allergic children. Clin. Exp. Allergy 33, 633–639 (2003).
Silver, E. et al. Lower levels of plasmacytoid dendritic cells in peripheral blood are associated with a diagnosis of asthma 6 yr after severe respiratory syncytial virus bronchiolitis. Pediatr. Allergy Immunol. 20, 471–476 (2009).
Son, M., Santiago-Schwarz, F., Al-Abed, Y. & Diamond, B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc. Natl. Acad. Sci. USA 109, 3160–3167 (2012).
Son, M. & Diamond, B. C1q-mediated repression of human monocytes is regulated by leukocyte-associated Ig-like receptor 1 (LAIR-1). Mol. Med. 20, 559–568 (2014).
de Heer, H.J. et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200, 89–98 (2004).
Sharma, M.D. et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Invest. 117, 2570–2582 (2007).
Tokita, D. et al. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation 85, 369–377 (2008).
Lewkowich, I.P. et al. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One 3, e3879 (2008).
Kohl, J. et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J. Clin. Invest. 116, 783–796 (2006).
Razafindratsita, A. et al. Improvement of sublingual immunotherapy efficacy with a mucoadhesive allergen formulation. J. Allergy Clin. Immunol. 120, 278–285 (2007).
Swedin, L. et al. Comparison of aerosol and intranasal challenge in a mouse model of allergic airway inflammation and hyperresponsiveness. Int. Arch. Allergy Immunol. 153, 249–258 (2010).
Barlow, J. L. et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 129, 191–198 (2012).
Acknowledgements
We thank Karine Jain for technical assistance in circular dichroism analysis. This work was funded by Stallergenes Greer.
Author contributions
L.M. conceived, supervised the study, and wrote the manuscript; S.A., N.B., C.Ga., and C.Gu. performed experiments in allergy models in vivo and in human models in vitro; G.F. performed experiments on Treg cell responses; B.B. and D.K. conceived and supervised experiments on Treg cell responses; D.T. and B.R. conceived and supervised experiment in the encephalomyelitis model in vivo; V.B.-B. supervised the study and critically reviewed the manuscript; P.M. supervised the study and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
L. Mascarell, S. Airouche, N. Berjont, C. Gueguen, V. Baron-Bodo, and P. Moingeon are employees at Stallergenes Greer. C. Gueguen was supported by a CIFRE fellowship from ANRT (Association Nationale de la Recherche et de la Technologie). D. Togbe is currently employed at ArtImmune and declares no conflict of interest. The remaining authors declare no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Mascarell, L., Airouche, S., Berjont, N. et al. The regulatory dendritic cell marker C1q is a potent inhibitor of allergic inflammation. Mucosal Immunol 10, 695–704 (2017). https://doi.org/10.1038/mi.2016.87
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.87
This article is cited by
-
LPS binding protein and activation signatures are upregulated during asthma exacerbations in children
Respiratory Research (2023)
-
Sex-dependent differences in the genomic profile of lingual sensory neurons in naïve and tongue-tumor bearing mice
Scientific Reports (2023)
-
Induction of PIR-A/B+ DCs in the in vitro inflammatory condition and their immunoregulatory function
Journal of Gastroenterology (2018)
-
Update on Biomarkers to Predict Responders to Allergen Immunotherapy
Current Treatment Options in Allergy (2017)