Abstract
The complete repair of the mucosa constitutes a key goal in inflammatory bowel disease (IBD) treatment. The Wnt signaling pathway mediates mucosal repair and M2 macrophages that coordinate efficient healing have been related to Wnt ligand expression. Signal transducer and activator of transcription 6 (STAT6) mediates M2 polarization in vitro and we hypothesize that a STAT6-dependent macrophage phenotype mediates mucosal repair in acute murine colitis by activating the Wnt signaling pathway. Our results reveal an impaired mucosal expression of M2 macrophage-associated genes and delayed wound healing in STAT6−/− mice treated with 2,4,6-trinitrobenzenesulfonic acid (TNBS). These mice also exhibited decreased mucosal expression of Wnt2b, Wnt7b, and Wnt10a, diminished protein levels of nuclear β-catenin that is mainly located in crypts adjacent to damage, and reduced mRNA expression of two Wnt/β-catenin target molecules Lgr5 and c-Myc when compared with wild-type (WT) mice. Murine peritoneal macrophages treated with interleukin-4 (IL-4) and polarized toward an M2a phenotype overexpressed Wnt2b, Wnt7b, and Wnt10a in a STAT6-dependent manner. Administration of a Wnt agonist as well as transfer of properly polarized M2a macrophages to STAT6−/− mice activated the Wnt signaling pathway in the damaged mucosa and accelerated wound healing. Our results demonstrate that a STAT6-dependent macrophage phenotype promotes mucosal repair in TNBS-treated mice through activation of the Wnt signaling pathway.
Similar content being viewed by others
INTRODUCTION
Inflammatory bowel disease (IBD) is a disorder of the gastrointestinal tract characterized by chronic inflammation at the submucosal level and disruption of epithelial barrier function. The complete repair of the epithelial layer is a key goal of current IBD treatment, as it has been related to long-term remission of this pathology.1, 2, 3, 4 Regeneration of the mucosa depends on a well-coordinated regulation between proliferation and differentiation into epithelial cell lineages of the progenitor cells, a process that is controlled principally by the Wnt signaling pathway.5, 6, 7 This pathway includes a group of ligands that act as intercellular signaling molecules that regulate cellular fate in normal gut epithelium and in response to epithelial injury. Upon binding to their receptors, canonical Wnt ligands induce inactivation of glycogen synthase kinase-3β and accumulation and nuclear translocation of β-catenin where it engages DNA-bound T-cell factor transcription factors.8, 9, 10, 11
Macrophages constitute an essential element of inflamed tissues, where they contribute to inflammatory injury and coordinate tissue repair. This variety of roles is possible because macrophages can adopt different functional phenotypes that differ in the expression of surface proteins and the production of cytokines.12, 13, 14 Two of the best characterized in vitro phenotypes are a proinflammatory M1 phenotype that mediates the defense of the host from microorganisms and the M2a phenotype that expresses high levels of antiinflammatory cytokines and scavenger molecules. M2a macrophages are frequently termed “wound healing macrophages” as they express factors that are important for tissue repair.15, 16, 17 Several studies have reported that activated macrophages express Wnt ligands,18, 19, 20 and we have recently demonstrated in cultured macrophages that M2a, but not M1, macrophages overexpress the canonical Wnt ligands Wnt1 and Wnt3a.21
In contrast to that reported in vitro, infiltrating macrophages do not conform to defined M1 or M2 phenotypes during tissue repair and adopt a continuum spectrum of functional phenotypes depending on the microenvironment. Previous studies that have analyzed the expression of M1 or M2 markers over time have reported the phenotypic transition of macrophages during skin and skeletal muscle repair,15, 16, 22 but there is limited information available regarding the switch during intestinal repair. The transcription factor signal transducer and activator of transcription 6 (STAT6) plays an essential role in M2a polarization induced by treatment with interleukin (IL)-4 or IL-13 in vitro.23, 24, 25 As would be expected, this pathway also promotes M2 macrophage polarization in vivo,26, 27, 28 although an IL-4/STAT6-independent M2 polarization has also been reported.17, 29, 30 In this study we aim to analyze the role of STAT6 in regulating the macrophage phenotype in the mucosa of 2,4,6-trinitrobenzenesulfonic acid (TNBS)-treated mice and the relevance of this pathway in intestinal repair. Our results demonstrate that STAT6 mediates the expression of M2 markers as well as Wnt2b, Wnt7b, and Wnt10a in the mucosa of TNBS-treated mice and show that a STAT6-dependent macrophage phenotype promotes mucosal repair through the activation of the Wnt signaling pathway.
RESULTS
STAT6 deficiency delays wound healing in a murine model of acute colitis
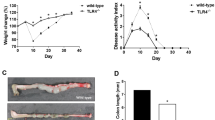
The role of STAT6 in the severity of colitis induced by TNBS was analyzed in wild-type (WT) and STAT6−/− mice. The STAT6−/− mice had a normal birth rate and similar weight gain and intestinal histology to WT mice. Treatment of WT mice with TNBS induced a loss of body weight, an increase in the histological damage score, and a diminution in colon length that peaked 2 days after treatment. Subsequently, mice began to recover and, 6 days after treatment, reached similar values to those of control animals in all these parameters. Changes in body weight, histological damage score, and colon length in STAT6−/− mice were similar 2 days after TNBS treatment. However, the recovery of these mice was delayed, as significant differences were observed with respect to WT mice in body weight gain, histological damage, and colon length 4 and 6 days after injury (Figure 1a–c). These results show that STAT6 deficiency significantly delays the functional recovery of mice and colonic wound healing in acute colitis.
Signal transducer and activator of transcription 6 (STAT6) deficiency delays wound healing in acute colitis induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS). Wild-type (WT) and STAT6−/− mice were treated with intrarectal administration of TNBS (3.5 mg per 20 g mice) or vehicle (Vh) and were killed 2, 4, and 6 days after treatment. Graphs show (a) body weight as a percentage of starting weight, measured daily after TNBS administration; (b) colon length after killing. (c) Histological score analyzed according to the Wallace score parameters and representative photographs of the mucosa after TNBS administration (original magnification × 10). Points or bars in the graphs represent mean±s.e.m. of at least 12 animals per experimental group. Significant differences in relation to the respective vehicle-treated group are shown by *P<0.05, **P<0.01, and ***P<0.001 and from the TNBS-WT mice by #P<0.05, ##P<0.01, and ###P<0.001.
The expression of M2 macrophage-associated genes in the colonic mucosa of TNBS-treated mice is STAT6 dependent
Real-time PCR of the colonic mucosa of vehicle-treated mice demonstrated that mRNA levels of M1 macrophage-associated genes (iNOS, Cd11c, CD86 and CCR7) and proinflammatory cytokines (TNFα, IL-1β, and IL-6) did not significantly differ between STAT6−/− and WT animals. Similar mRNA expression of M2 macrophage-associated genes (CD206, ArgI, Fizz1, and Ym1) and antiinflammatory cytokines (IL-10 and IL-13) was also observed between STAT6−/− and WT animals, except for IL-4 that was significantly decreased in knockout animals compared with WT mice (Supplementary Figure S1 online).
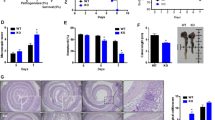
In WT mice, treatment with TNBS increased the mucosal mRNA expression of proinflammatory cytokines (TNFα, IL-1β, and IL-6) and M1 markers (iNOS, CD11c, CD86, and CCR7), although the magnitude and temporal pattern of expression slightly differed between genes (Figure 2a,b). Expression of TNFα, IL-1β, iNOS, and CD11c peaked 2 days after TNBS treatment, whereas IL-6, CD86, and CCR7 peaked 4 days after injury. Nonsignificant differences were detected between WT and STAT6−/− animals, except for the expression of iNOS, TNFα, and IL-6 that dropped in WT mice whereas it remained high in STAT6−/− mice. The analysis of M2 markers and antiinflammatory cytokines in WT animals showed an early increase in the mRNA expression of Ym1 and ArgI, whereas the expression of CD206 peaked 4 days after injury and Fizz1, IL-10, IL-4, and IL-13 did at day 6. Of interest, TNBS failed to induce the expression of CD206, Fizz1, IL-10, IL-4, and IL-13 in STAT6−/− mice and induced a lower expression of ArgI and Ym1 in STAT6−/− than in WT mice (Figure 2a,b).
Signal transducer and activator of transcription 6 (STAT6) regulates the expression of M2 macrophage-associated genes in the mucosa of 2,4,6-trinitrobenzenesulfonic acid (TNBS)-treated mice. Wild-type (WT) and STAT6−/− mice were treated with TNBS or vehicle (Vh) and were killed 2, 4, and 6 days (2d, 4d, and 6d) after treatment. (a) Graphs show relative mRNA expression levels of proinflammatory and antiinflammatory cytokines in the mucosa vs. the housekeeping gene β-actin and represented as fold induction vs. vehicle WT group (horizontal dot line) (n=6). (b) Graphs show relative mRNA expression levels of M1 markers (CD86, CCR7, CD11c, and iNOS) and M2 markers (CD206, Ym1, Fizz1, and ArgI) in the mucosa vs. the housekeeping gene β-actin and represented as fold induction vs. vehicle WT group (horizontal dot line) (n=6). (c) Immunohistochemistry for CCR7- and CD206-positive cells was performed in the mucosa 4 days after TNBS treatment and graphs show the quantification of these cells in a total area of 0.3 mm2 (n=6). (d) Representative photographs of CCR7 and CD206 immunostaining in WT mice (original magnification × 40). In all cases, bars represent mean±s.e.m. Significant differences from the respective vehicle-treated group are shown by *P<0.05, **P<0.01, and ***P<0.001 and from the TNBS-WT mice by #P<0.05, ##P<0.01, and ###P<0.001.
Immunohistochemical studies revealed the presence of CCR7- and CD206-positive cells in the lamina propria of the murine intestinal mucosa. A quantitative analysis indicated that the number of CCR7-positive cells was higher in the mucosa of TNBS- than vehicle-treated animals at 2 days after injury. This number remained high at day 4 but fell at day 6, with a similar number of positive cells being detected in treated and control animals. Similar results were observed in STAT6−/− mice 2 and 4 days after injury. However, the number of CCR7-positive cells at 6 days after TNBS was significantly higher in STAT6−/− than in WT mice (Figure 2c).
The number of CD206-positive cells in the mucosa of WT mice was similar in vehicle- and TNBS-treated mice at 2 days after injury, but a significant increase was observed at day 4 that was maintained until 6 days after treatment. In contrast, the number of CD206-positive cells in the mucosa of STAT6−/− mice was not significantly increased by TNBS at any of the time points analyzed (Figure 2c). As a whole, our results demonstrate that STAT6 mediates the expression of M2, and not M1, macrophage-associated genes in the damaged mucosa of TNBS-treated mice.
STAT6 deficiency prevents the increase in the Wnt signaling pathway detected in the mucosa of TNBS-treated mice
The canonical Wnt/β-catenin signaling pathway plays an essential role in mucosal regeneration following intestinal injury. The study of mRNA expression of canonical Wnt ligands in the mucosa of Balb/c mice revealed the presence of Wnt2b, Wnt6, Wnt7b, Wnt10a, and Wnt10b, whereas Wnt1 and Wnt3a were not detected (Figure 3a). All these genes were also detected in cells of the lamina propria, whereas only Wnt2b, Wnt6, and Wnt10b were expressed in intestinal epithelial cells from the mucosa. The analysis of the basal expression of these genes in the mucosa revealed nonsignificant differences between WT and STAT6−/− mice. At 4 days after TNBS administration to WT mice, we found an increased expression of Wnt2b, Wnt7b, and Wnt10a in both the mucosa and cells of the lamina propria and an increased expression of Wnt2b in epithelial cells. The expression of Wnt6 and Wnt10b was not significantly altered by TNBS treatment in any cell type analyzed (Figure 3a). In STAT6−/− mice, treatment with TNBS failed to significantly induce Wnt2b, Wnt7b, and Wnt10a mRNA expression in both the mucosa and cells of the lamina propria, whereas it significantly increased Wnt2b expression in epithelial cells, as happened in WT mice. (Figure 3a).
Signal transducer and activator of transcription 6 (STAT6) regulates the expression of Wnt ligands in cells of lamina propria. Wild-type (WT) and STAT6−/− mice were treated with 2,4,6-trinitrobenzenesulfonic acid (TNBS) and were killed 4 days after treatment. (a) Representative photograph showing the presence of canonical Wnt ligands in the mucosa, lamina propria cells, or epithelial cells from Balb/c mice (n=3). Graphs show quantification of Wnt2b, Wnt6, Wnt7b, Wnt10a, and Wnt10b mRNA in the intestinal mucosa, lamina propria cells, and epithelial cells (n=5). (b) Graphs show mRNA expression levels of DKK1 and SFRP1 in the mucosa 4 days after treatment with TNBS. (c) Graph shows protein expression of DKK1 in mucosa 4 days after treatment with TNBS (n=5). Photograph of a representative western blot. In all cases, bars represent mean±s.e.m. and significant differences from the respective vehicle (Vh)-treated group are shown by *P<0.05, **P<0.01, and ***P<0.001 and from the TNBS-WT mice by #P<0.05, ##P<0.01, and ###P<0.001.
The analysis of the mRNA and protein expression of DKK1, as well as the mRNA expression of SFRP1, the two negative regulators of Wnt, revealed a significant increase in the mucosa of TNBS-treated mice compared with that of vehicle-treated mice. Nonsignificant differences in the expression of these negative regulators were detected between WT and STAT6−/− mice (Figure 3b,c).
Immunohistochemical studies revealed the presence of β-catenin in epithelial cells of the mucosa of TNBS-treated animals. Nuclear expression of this protein was mainly observed in crypts adjacent to damage (Figure 4a). Western blot quantification of the amount of nuclear β-catenin revealed higher protein levels in the mucosa of TNBS-treated mice than in that of vehicle animals 4 days after injury (Figure 4b). Furthermore, TNBS also induce the mRNA expression of two target genes, c-Myc and Lgr5, in WT mice (Figure 4c). The increase in nuclear β-catenin induced by TNBS was not observed in the mucosa of STAT6−/− mice, in which a significant reduction in the mRNA expression of two target genes, c-Myc and Lgr5, was also detected with respect to WT mice (Figure 4c). These results show the activation of the Wnt signaling in the damaged mucosa of TNBS-treated WT mice and reveal that this pathway is impaired in the damaged mucosa of STAT6−/− animals.
Signal transducer and activator of transcription 6 (STAT6) deficiency prevents the increase in the mucosal Wnt signaling pathway induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS). Wild-type (WT) and STAT6−/− mice were treated with TNBS and were killed 4 days after treatment. (a) Representative photographs showing β-catenin immunostaining in paraffin-embedded colons (original magnifications × 10, × 20, and × 40); n=4). (b) A representative western blot of nuclear β-catenin and nucleolin. Graph shows the quantification of nuclear β-catenin normalized with nucleolin (n=4). (c) Graphs show the mRNA expression of two Wnt/β-catenin target genes, Lgr5 and c-Myc (n=4). In all cases, bars represent mean±s.e.m. and significant differences from the respective vehicle (Vh)-treated group are shown by *P<0.05 and **P<0.01 and from the TNBS-WT mice by #P<0.05, ##P<0.01, and ###P<0.001. (d) Graph shows a positive and significant correlation between the number of CD206-positive cells and nuclear β-catenin protein levels normalized with nucleolin in the mucosa of TNBS-treated mice.
Interestingly, analysis of nuclear β-catenin protein levels and the number of CD206-positive cells in the mucosa of WT and STAT6−/− mice treated with TNBS highlighted a positive and significant correlation between these two parameters (Figure 4d), leading us to suggest that M2 macrophages modulate the Wnt signaling pathway in damaged mucosa.
STAT6 mediates Wnt ligands expression in M2a macrophages
Peritoneal murine macrophages were obtained from WT and STAT6−/− mice and polarized into M1 or M2a macrophages in vitro by administration of lipopolysaccharide+interferon-γ (LPS+IFN-γ) or IL-4, respectively. An increase in the mRNA expression of M1 markers (iNOS and CD86) and proinflammatory cytokines (TNFα, IL-1β, and IL-6) was observed in macrophages from both WT and STAT6−/− mice treated with LPS+IFN-γ. Administration of IL-4 enhanced the mRNA expression of CD206, ArgI, and IL-10 in macrophages from WT mice, but it was not observed in macrophages obtained from STAT6−/− mice, reinforcing previous evidence that STAT6 is required for in vitro M2a polarization induced by IL-4 (Figure 5a,b).
M2a macrophages overexpressed Wnt ligands through signal transducer and activator of transcription 6 (STAT6). Murine peritoneal macrophages were obtained and polarized into M1 and M2a macrophages with lipopolysaccharide+interferon-γ (LPS+IFN-γ) or interleukin-4 (IL-4), respectively. (a) Graphs show mRNA expression of iNOS, CD86, CD206, and ArgI in macrophages (n=4). (b) Graphs show mRNA expression of TNFα, IL-1β, IL-6, and IL-10 in macrophages (n=4). (c) Representative photograph showing the presence of canonical Wnt ligands in peritoneal macrophages obtained from mice (n=3). Graphs show the quantification of Wnt2b, Wnt7b, and Wnt10a mRNA expression in macrophages (n=4). In all cases, results were normalized with β-actin and are represented as fold induction vs. nonpolarized macrophages. Significant differences from respective nonpolarized macrophages are shown by *P<0.05, **P<0.01, and ***P<0.001 and from M2a macrophages obtained from WT mice by #P<0.05 and ###P<0.001. (d) Caco-2 cells were cocultured 24 h with peritoneal macrophages from WT and STAT6−/− mice polarized toward M2a macrophages and protein expression of nuclear β-catenin in Caco-2 cells was analyzed. Results were normalized with nucleolin. A representative western blot is shown. Bars in graphs represent mean±s.e.m. Significant differences from Caco-2 cells cocultured with nonpolarized macrophages from WT are shown by ***P<0.001 and from M2-macrophages from WT by ###P<0.001.
The study of mRNA expression of canonical Wnt ligands in peritoneal macrophages from WT mice revealed the presence of Wnt2b, Wnt7b, Wnt10a, and Wnt10b (Figure 5c). Polarization of these cells toward an M2a and not M1 phenotype was associated with increased mRNA expression of Wnt2b, Wnt7b, and Wnt10a, whereas Wnt10b was not significantly modified. The expression of these genes in nonpolarized macrophages was not significantly different between WT and STAT6−/− mice. However, no induction of these genes was observed in STAT6−/− macrophages treated with IL-4, showing that STAT6 mediates the expression of Wnt ligands associated with M2a macrophage polarization (Figure 5c).
Macrophages obtained from WT mice and polarized toward an M2a phenotype increased the nuclear β-catenin expression in epithelial cells in coculture compared with nonpolarized macrophages (Figure 5d). In contrast, macrophages obtained from STAT6−/− mice did not significantly modify nuclear β-catenin protein levels in epithelial cells (Figure 5d). As a whole, the result demonstrates that STAT6 mediates the expression of canonical Wnt ligands in M2a macrophages that activate Wnt signaling in intestinal epithelial cells.
Transfer of M2a macrophages activates Wnt signaling pathway and accelerates wound healing in the mucosa of STAT6−/− TNBS-treated mice
We next aimed to analyze whether the administration of M2a macrophages may modulate mucosal Wnt signaling and wound healing in STAT6−/− mice. First, in order to study the importance of this pathway in mucosal healing, we administered a Wnt agonist to TNBS-treated STAT6−/− mice and the results showed an accelerated recovery of body weight (Figure 6a), an enhancement of the colon length (Figure 6b), and a reduction in the histological damage score at 4 days after TNBS (Figure 6c). All these changes paralleled with a significant increase in nuclear β-catenin protein levels in the colonic mucosa (Figure 6d) and suggest that activation of mucosal Wnt signaling is associated with acceleration of wound healing.
Treatment with a Wnt agonist accelerates wound healing in TNBS-induced colitis in STAT6−/− mice. STAT6−/− mice treated with TNBS received daily an intraperitoneal injection of a Wnt agonist (5 mg kg−1) until day 4. (a) Graph shows body weight as a percentage of starting weight, measured daily after TNBS administration. (b, c) Graphs show colon length after killing and histological score analyzed according to the Wallace score parameters. Representative photographs of the mucosa after TNBS administration (original magnification × 10). (d) Graph shows quantification of nuclear β-catenin vs. nucleolin and results are represented as fold induction. A representative western blot showing nuclear β-catenin. In all cases, points or bars in graphs represent mean±s.e.m. Significant differences in relation to vehicle (Vh)-treated group are shown by *P<0.05 and **P<0.01. STAT6, signal transducer and activator of transcription 6; TNBS, 2,4,6-trinitrobenzenesulfonic acid.
Finally, macrophages obtained from WT and STAT6−/− mice and treated with IL-4 were administered intraperitoneally to STAT6−/− mice at 2 days after TNBS treatment. Immunolocalization studies revealed the presence of these cells in the colon, with no differences in the accumulation of either cell type. As shown in Figure 7a, the rate of recovery in body weight of mice receiving macrophages from WT animals was higher than in those receiving macrophages from STAT6−/− mice. Analysis of the mucosa at day 4 revealed an increased colon length (Figure 7b), lower histological damage score (Figure 7b,c), and decreased mRNA expression of iNOS, TNFα, and IL-1β (Figure 7d) in mice receiving macrophages from WT animals than in those receiving macrophages from STAT6−/− mice. A quantitative analysis showed that the exogenous administration of macrophages obtained from WT mice significantly increased nuclear protein levels of β-catenin compared with administration of macrophages from STAT6−/− animals (Figure 7e). In line with this, the mucosal mRNA expression of Lgr5 and c-Myc was higher in the mucosa of mice treated with macrophages from WT mice than in those receiving macrophages from STAT6−/− mice (Figure 7f). As a whole, these results suggest that the administration of M2a macrophages, which signal through STAT6, accelerates the mucosal repair in TNBS-treated STAT6−/− mice by the activation of the Wnt signaling pathway.
Transfer of M2a macrophages accelerate wound healing in STAT6−/− 2,4,6-trinitrobenzenesulfonic acid (TNBS)-treated mice through the activation of the Wnt signaling pathway. Peritoneal macrophages from wild-type (WT) and from STAT6−/− mice were polarized toward M2a macrophages with interleukin-4 (IL-4) and injected intraperitoneally (i.p.; 2 × 106 macrophages) 2 days after TNBS administration. Mice were killed 2 days after macrophage injection. (a) Graph shows body weight as a percentage of starting weight measured daily after TNBS administration (n=5). (b) Graphs show colon length and histological score 2 days after macrophage injection (n=5). (c) Representative photographs of the mucosa of TNBS-treated mice that received macrophages (original magnification × 10). (d) Graphs show mRNA expression of iNOS, TNFα, and IL-1β in the mucosa of mice receiving macrophages. Results were normalized with β-actin and are represented as fold induction vs. expression in the group of mice treated with macrophages obtained from STAT6−/− mice. (e) Graph shows the densitometry quantification of nuclear β-catenin normalized with nucleolin (n=5) and a representative western blot is also shown. (f) Graphs show the expression of c-Myc and Lgr5 normalized with β-actin and represented as fold induction vs. expression in mice receiving macrophages obtained from STAT6−/− mice (n=5). In all cases, bars in graphs represent mean±s.e.m. and significant differences from the group of mice treated with macrophages obtained from STAT6−/− mice are shown by *P<0.05, **P<0.01, and ***P<0.001. STAT6, signal transducer and activator of transcription 6.
DISCUSSION
This study demonstrates that a STAT6-dependent macrophage phenotype mediates mucosal repair in TNBS-treated mice through the activation of the Wnt signaling pathway.
The experimental model of colitis induced by a single administration of TNBS to Balb/c mice was characterized by loss of body weight, distortion of mucosal architecture, and dense infiltration of macrophages, similar characteristics to those observed in human Crohn’s disease.31 These changes peaked 2 days after injury and were followed by efficient tissue repair and functional recovery. Our data demonstrate that the absence of STAT6 delays regeneration of the mucosa and the subsequent recovery of mice. These observations are in line with previous studies showing exacerbation of chronic32 or acute colitis33 in STAT6−/− mice, but contrast with those reported by Rosen et al.,34 who observed amelioration of oxazolone-induced colitis. This is an expected exception because in this particular model, but not in the TNBS model, colitis depends on IL-13 secretion and the consequent STAT6 activation,35, 36, 37 a pathway that has been described as a possible pathogenic mechanism of human colitis, but irrelevant in Crohn’s disease.38, 39
Macrophages are essential to efficient healing as they promote clearance of debris, cell proliferation, angiogenesis, collagen deposition, and matrix remodeling.40, 41, 42 The diversity of the roles played by macrophages is because of their ability to assume a continuum spectrum of functional phenotypes.12, 15, 22 Our results reveal increased expression of both M1 and M2 markers and proinflammatory and antiinflammatory cytokines in the damaged mucosa of WT mice treated with TNBS. The early expression observed in iNOS and CD11c, as well as that of two hallmark indicators of M2 activation (ArgI and Ym1), suggests that macrophages in the inflammatory phase of intestinal repair adopt a combination of M1 and M2 phenotypes similar to that reported in other tissues.15, 16, 43 At 4 days after TNBS, there was a reduction in the expression of iNOS, CD11c, and Ym1 in parallel with a peak in the expression of CD86, CCR7, CD206, and ArgI, and an increased expression of antiinflammatory cytokines, suggesting a different phenotypic profile than that observed in the inflammatory phase. At 6 days after TNBS, when a functional and histological recovery was observed in WT mice, low levels in M1- and sustained levels in M2-macrophage-associated genes were recorded, pointing to the fact that macrophages had been properly activated to repair the mucosa. This assumption is supported by the different time course of macrophage activation subject to STAT6 deficiency. A higher expression of proinflammatory cytokines together with a lower expression of both M2 markers and antiinflammatory cytokines coexisted with a delayed wound healing in STAT6−/− mice. Interestingly, this defect was counteracted by exogenous administration of effectively polarized M2a macrophages obtained from WT mice but not by macrophages obtained from STAT6−/− mice in which M2a polarization had failed. The beneficial effects of administration of M2a macrophages to STAT6−/− mice were also evident in the functional recovery of mice and the diminished mucosal expression of proinflammatory markers. As a whole, our results indicate that a STAT6-dependent macrophage phenotype mediates mucosal repair in TNBS-treated mice.
During tissue repair, the proliferation of numerous cell types is a requirement of normal healing. In the gastrointestinal tract, the Wnt signaling pathway mediates regeneration of the mucosa by activating proliferation of the progenitor cells located at the base of the crypts.10, 11, 44
Our results reveal in the mucosa of TNBS-treated mice an accumulation of nuclear β-catenin, the central player in the canonical Wnt pathway, and increased transcriptional expression of two Wnt target genes, Lgr5 and c-Myc, indicating that upregulation of the canonical Wnt pathway is an injury-associated response.19 Of interest, nuclear β-catenin was mainly located in crypts adjacent to injury, strongly supporting that activation of Wnt signaling in epithelial cells takes place to renew the damaged area.11, 14 This is strongly reinforced by results showing activation of Wnt signaling and acceleration of mucosal healing with the administration of a Wnt agonist to STAT6−/− mice that had exhibited a delayed wound healing associated with a diminished activation of this pathway.
Regulation of the Wnt signaling pathway is complex and may occur at multiple levels, including the ligand/receptor, translocation of β-catenin, and transcriptional activation.9 Our results showed an increase in the expression of canonical Wnt ligands in the mucosa of TNBS-treated mice. We also detected an enhanced expression of negative regulators of Wnt that have been reported to be expressed during intestinal inflammation.45 Of interest, data revealed that the expression of Wnt ligands, but not that of the Wnt inhibitors, is mediated by STAT6, pointing to upregulation of Wnt ligands expression in the activation of mucosal Wnt signaling. In response to injury, these ligands were upregulated in both cells of the lamina propria and epithelial cells. However, this regulation was dependent on STAT6 only in cells of the lamina propria, suggesting that differences detected in mucosal levels of β-catenin between WT and STAT6 knockout mice are because of the expression of Wnt ligands in cells other than epithelial cells.
Macrophages have been related with the expression of Wnt ligands,21 and it has been reported that activated macrophages promote Wnt signaling in epithelial cells in the kidney and stomach, as well as in cocultured intestinal epithelial cells.19, 21, 46, 47 The present study extends these observations and show that both the expression of Wnt ligands by M2a macrophages and the activation of epithelial Wnt signaling by cocultured M2a macrophages are STAT6 dependent. These results help to explain the deficiency in Wnt signaling detected in the mucosa of STAT6−/− mice and point to M2 macrophages as important modulators of Wnt signaling in damaged mucosa. Strong evidence of the role played by M2a macrophages in activating the Wnt pathway comes from results showing that the injection of M2a macrophages to STAT6−/− mice increased the amount of nuclear β-catenin and mRNA expression of two Wnt/β-catenin target genes and accelerated wound repair in the mucosa of TNBS-treated mice. This acute response, which seems to be required for wound healing, needs to be balanced to avoid a mucosal dysfunction because of the persistence of Wnt signaling associated with accumulation of M2 macrophages, as seen in chronic ulcerative colitis patients.21
In summary, our study demonstrates that a STAT6-dependent macrophage phenotype promotes mucosal repair in TNBS-treated mice through activation of the Wnt signaling pathway. A better understanding of the reciprocal regulation of macrophage phenotype and mucosal repair following intestinal damage would help to establish new cellular approaches to IBD therapy.
METHODS
Mice
WT Balb/cj and STAT6−/− knockout (C.129S2-Stat6tm1Gru/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and kept under specific pathogen-free conditions. Experiments were performed when mice were 7–8 weeks old. All protocols were approved by the institutional animal care and use committees of the University of Valencia (Valencia, Spain).
Induction and evaluation of colitis
TNBS colitis was induced by intrarectal administration of 100 μl of TNBS (3.5 mg per 20 g mice) dissolved in 40% ethanol. Mice were anesthetized and a 16G catheter was then carefully inserted through the anus into the colon. The catheter was introduced 3 cm from the anus and mice were hung upside down for 1 min. Vehicle-treated mice received 100 μl of 0.9% NaCl dissolved in 40% ethanol. Mice were weighed daily and were killed by cervical dislocation on days 2, 4, and 6 after TNBS administration. Colon length was measured and colon tissue was frozen in liquid nitrogen for protein and RNA extraction and fixed in 4% paraformaldehyde acid and embedded in paraffin for immunohistochemistry experiments. Some mice were treated daily with a Wnt agonist (5 mg kg−1, CID 11210285 hydrochloride, Sigma-Aldrich, St Louis, MO) or its vehicle (dimethyl sulfoxide 5%) intraperitoneally. Histological analysis was performed on a scale of 0 to 10, taking into account the parameters of the Ameho Criteria48 that are degree of inflammatory infiltrate, the presence of erosion, ulceration or necrosis, and the depth and surface extension of lesions.
Isolation of peritoneal macrophages and in vitro polarization
Mice were killed and 10 ml of cold phosphate-buffered saline was injected into the peritoneal cavity. The fluid was then withdrawn and centrifuged for 5 min at 350 g at 4 °C. The pellet was resuspended in 1 ml of RPMI-1640 (HiClone, GE Healthcare Life Science, South Logan, UT) supplemented with 2% penicillin–streptomycin and 10% bovine fetal serum These cells were seeded in Petri dishes for 4 h at 37 °C. Nonadherent cells were subsequently removed by washing with phosphate-buffered saline and adherent macrophages were polarized toward M1 macrophages with lipopolysaccharide (0.1 μg ml−1; Escherichia coli 0111:B4) and interferon-γ (20 ng ml−1) for 24 h or toward M2a macrophages with IL-4 (20 ng ml−1) for 72 h.49 Macrophages were characterized by reverse transcription–PCR for iNOS, CD86, ArgI, and CD206.
Transfer of M2a macrophages
M2a macrophages derived from peritoneal macrophages were labeled with Vybrant Cell-Labeling Solutions (Molecular Probes, Eugene, OR) according to the manufacturer’s instructions. In brief, cells were removed by incubation with TrypLE Express (Invitrogen, Waltham, MA) and EDTA (1 mM) in Dulbecco’s phosphate-buffered saline at 37 °C for 30 min. They were then resuspended at a density of 1.0 × 106 cells and incubated for 20 min with Vybrant Cell-Labeling Solution. Cells were washed with warm medium twice and 2.0 × 106 macrophages were injected intraperitoneally with a 19G needle 2 days after TNBS administration. Mice were killed 2 days after macrophage injection.
Epithelial and lamina propria cell isolation
Mice were killed 4 days after TNBS administration and fresh colons were removed and washed with Hanks’ balanced salt solution without Ca+2 or Mg+2. Colons were cut and incubated with 3 mM EDTA and 2 mM dithiothreitol (1 h, 4 °C). Supernatant was collected and epithelial cells were obtained after centrifugation (5 min, 1,500 r.p.m.).47 Intestinal pieces were cut and incubated with Collagenase IV (1 mg ml−1, Life Technologies, Carlsbad, CA) and DNAse (0.3 mg ml−1, GE Healthcare, Milwaukee, WI) for 30 min at 37 °C. Mucosa was dissociated with a gentle MACS dissociator (Miltenyi Biotec, Madrid, Spain) and filtered with 70 μm. Lamina propria cells were collected after centrifugation (10 min, 1,500 r.p.m).
Cell culture and coculture
Caco-2 cells (American Type Culture Collection, Manassas, VA) were cultured in minimum essential medium (Sigma-Aldrich) supplemented with 20% inactivated bovine fetal serum, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 2 mM L-glutamine, 100 mM sodium pyruvate, and 1% of non-essential amino acids. These cells were cocultured with peritoneal macrophages from WT and STAT6−/− mice using Transwell inserts (Corning, Springfield, MA) with a 0.4 μm porous membrane.21 Peritoneal macrophages were seeded on the inserts and polarized toward the M2 phenotype as described above. After polarization, the inserts were placed on top of a monolayer of Caco-2 cells and were cocultured for 24 h.
Protein extraction and western blot
Intestinal frozen tissues were homogenized and nuclear and cytoplasm protein was extracted as previously described.21 Nuclear protein from Caco-2 cells was obtained by sonication of nuclear pellets followed by centrifugation. Equal amounts of protein were loaded onto sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels. After electrophoresis, membranes were blocked with 5% non-fat dry milk in TBS-T (20 mM Tris/HCl pH 7.2, 150 mM NaCl, and 0.1% Tween-20) and incubated overnight with different primary antibodies (Table 1). Later, membranes were incubated with a peroxidase-conjugated anti-mouse IgG (1:2,500; Thermo Scientific, Rockford, IL) or anti-rabbit IgG (1:5,000; Thermo Scientific) followed by treatment with supersignal west pico chemiluminescent substrate (Thermo Scientific). Protein bands were detected by a LAS-3000 (Fujifilm, Barcelona, Spain) and protein expression was quantified by means of densitometry using Image Gauge Version 4.0 software (Fujifilm). Data were normalized to β-actin for total and cytoplasm proteins or nucleolin for nuclear proteins.
Immunohistochemistry
Intestinal tissues were fixed in 10% paraformaldehyde and immunohistochemical studies were performed in representative 5 μm sections of paraffin-embedded tissues. After dehydration of the tissues, endogenous peroxidase activity was suppressed by immersion in 0.3% hydrogen peroxide (15 min), after which antigen retrieval was performed. Following blocking with 5% rabbit or goat serum for 30 min, sections were incubated overnight (4 °C) with different primary antibodies (Table 1). Tissues were incubated with anti-mouse IgG (1:200; Thermo Scientific) or anti-rabbit IgG (1:200; Thermo Scientific) and 3,3′-diaminobenzidine was employed for signal development. All tissues were counterstained with hematoxylin and the specificity of the immunostaining was confirmed by the absence of staining in analogous tissue sections when the primary or secondary antibodies were omitted. Quantification of CCR7+ cells and CD206+ cells was performed in a total area of 0.3 mm2.
RNA extraction and real-time PCR analysis
RNA extraction was performed using Tripure Isolation reagent (Roche, Barcelona, Spain). In short, tissues were homogenized by Ultraturrax (Sigma-Aldrich) in 750 μl Trizol, and epithelial cells, cells from the lamina propria, or peritoneal macrophages were suspended in 750 μl Trizol. Afterward, 200 μl chloroform was added to the separation phase. RNA precipitation was performed by adding 500 μl isopropanol, and RNA pellets were washed twice with 70% ethanol. RNA was quantified with Nanodrop (Thermo Scientific) and 1 μg of RNA was used to obtain complementary DNA with the Prime Script RT reagent Kit (Takara Biotechnology, Dalian, China). Real-time PCR was performed with the Prime Script Reagent Kit Perfect Real Time (Takara Biotechnology) in a thermo cycler Light Cycler (Roche Diagnostics, Mannheim, Germany). Specific oligonucleotides were designed according to the sequences shown in Table 2. Relative gene expression was expressed as follows: change in expression (fold)=2−Δ(ΔCT) where ΔCT=CT (target)−CT (housekeeping), and Δ(ΔCT)=ΔCT (treated)−ΔCT (control). β-Actin was used as the housekeeping gene. PCR products of Wnt ligands were loaded onto an agarose gel and bands were detected by a LAS-3000 (Fujifilm).
Statistical analysis
Data were expressed as mean±s.e.m. and compared by analysis of variance (one-way analysis of variance) with Newman–Keuls post hoc correction for multiple comparisons or unpaired Student’s t-test where appropriate. A P value of <0.05 was considered to be statistically significant. Correlations were analyzed using Spearman’s correlation coefficient.
References
Neurath, M.F. & Travis, S.P. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 61, 1619–1635 (2012).
Maloy, K.J. & Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 (2011).
Henderson, P., van Limbergen, J.E., Schwarze, J. & Wilson, D.C. Function of the intestinal epithelium and its dysregulation in inflammatory bowel disease. Inflamm. Bowel Dis. 17, 382–395 (2011).
Bernstein, C.N. Treatment of IBD: where we are and where we are going. Am. J. Gastroenterol. 110, 114–126 (2015).
Pinto, D., Gregorieff, A., Begthel, H. & Clevers, H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17, 1709–1713 (2003).
van de Wetering, M. et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002).
van der Flier, L.G. & Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241–260 (2009).
Yeung, T.M., Chia, L.A., Kosinski, C.M. & Kuo, C.J. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell. Mol. Life Sci. 68, 2513–2523 (2011).
Clevers, H. & Nusse, R. Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205 (2012).
Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284 (2013).
Clevers, H., Loh, K.M. & Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012 (2014).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Murray, P.J. & Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Pull, S.L., Doherty, J.M., Mills, J.C., Gordon, J.I. & Stappenbeck, T.S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA 102, 99–104 (2005).
Novak, M.L. & Koh, T.J. Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 93, 875–881 (2013).
Gensel, J.C. & Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619, 1–11 (2015).
Daley, J.M., Brancato, S.K., Thomay, A.A., Reichner, J.S. & Albina, J.E. The phenotype of murine wound macrophages. J. Leukoc. Biol. 87, 59–67 (2010).
Boulter, L. et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 18, 572–579 (2012).
Lin, S.L. et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. USA 107, 4194–4199 (2010).
Smith, K. et al. Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br. J. Cancer 81, 496–502 (1999).
Cosin-Roger, J. et al. M2 macrophages activate WNT signaling pathway in epithelial cells: relevance in ulcerative colitis. PLoS One 8, e78128 (2013).
Novak, M.L. & Koh, T.J. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 183, 1352–1363 (2013).
Mikita, T., Campbell, D., Wu, P., Williamson, K. & Schindler, U. Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol. Cell. Biol. 16, 5811–5820 (1996).
Egan, B.S., Abdolrasulnia, R. & Shepherd, V.L. IL-4 modulates transcriptional control of the mannose receptor in mouse FSDC dendritic cells. Arch. Biochem. Biophys. 428, 119–130 (2004).
Welch, J.S. et al. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J. Biol. Chem. 277, 42821–42829 (2002).
Weng, M. et al. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J. Immunol. 179, 4721–4731 (2007).
Odegaard, J.I. et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
D'Alessio, S. et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Invest. 124, 3863–3878 (2014).
He, L. & Marneros, A.G. Macrophages are essential for the early wound healing response and the formation of a fibrovascular scar. Am. J. Pathol. 182, 2407–2417 (2013).
Maier, E., Duschl, A. & Horejs-Hoeck, J. STAT6-dependent and -independent mechanisms in Th2 polarization. Eur. J. Immunol. 42, 2827–2833 (2012).
Wirtz, S. & Neurath, M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 59, 1073–1083 (2007).
Fichtner-Feigl, S. et al. IL-13 orchestrates resolution of chronic intestinal inflammation via phosphorylation of glycogen synthase kinase-3beta. J. Immunol. 192, 3969–3980 (2014).
Elrod, J.W. et al. DSS-induced colitis is exacerbated in STAT-6 knockout mice. Inflamm. Bowel Dis. 11, 883–889 (2005).
Rosen, M.J. et al. STAT6 deficiency ameliorates severity of oxazolone colitis by decreasing expression of claudin-2 and Th2-inducing cytokines. J. Immunol. 190, 1849–1858 (2013).
Heller, F., Fuss, I.J., Nieuwenhuis, E.E., Blumberg, R.S. & Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 17, 629–638 (2002).
Boirivant, M., Fuss, I.J., Chu, A. & Strober, W. Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J. Exp. Med. 188, 1929–1939 (1998).
Hoving, J.C. et al. B cells that produce immunoglobulin E mediate colitis in BALB/c mice. Gastroenterology 142, 96–108 (2012).
Geremia, A., Biancheri, P., Allan, P., Corazza, G.R. & Di, S.A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 13, 3–10 (2014).
Monteleone, G., Caruso, R. & Pallone, F. Targets for new immunomodulation strategies in inflammatory bowel disease. Autoimmun. Rev. 13, 11–14 (2014).
Wynn, T.A., Chawla, A. & Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013).
Mahida, Y.R. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm. Bowel Dis. 6, 21–33 (2000).
Mowat, A.M. & Bain, C.C. Mucosal macrophages in intestinal homeostasis and inflammation. J. Innate Immun. 3, 550–564 (2011).
Atochina, O., Da'dara, A.A., Walker, M. & Harn, D.A. The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo. Immunology 125, 111–121 (2008).
Miyoshi, H., Ajima, R., Luo, C.T., Yamaguchi, T.P. & Stappenbeck, T.S. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science 338, 108–113 (2012).
Nava, P. et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity 32, 392–402 (2010).
Oguma, K. et al. Activated macrophages promote Wnt signaling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 27, 1671–1681 (2008).
Ortiz-Masia, D. et al. Hypoxic macrophages impair autophagy in epithelial cells through Wnt1: relevance in IBD. Mucosal Immunol. 7, 929–938 (2013).
Ameho, C.K. et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut 41, 487–493 (1997).
Hunter, M.M. et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology 138, 1395–1405 (2010).
Acknowledgements
This work was supported by Ministerio de Economía y Competitividad (SAF2013-43441-P), CIBERehd, and Generalitat Valenciana [PROMETEOII/2014/035]. J.C.-R. is supported by FPU fellowships from Ministerio de Educación, Cultura y Deporte. C.H. acknowledges support from the ‘Ramon y Cajal’ programme of Spain. We thank Brian Normanly for his English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Cosín-Roger, J., Ortiz-Masiá, D., Calatayud, S. et al. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol 9, 986–998 (2016). https://doi.org/10.1038/mi.2015.123
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2015.123
This article is cited by
-
Experimental evidence of shikonin as a novel intervention for anti-inflammatory effects
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Loss of Dact2 alleviates cisplatin-induced nephrotoxicity through regulation of the Igfl-MAPK pathway axis
Cell Biology and Toxicology (2023)
-
Ketone body β-hydroxybutyrate ameliorates colitis by promoting M2 macrophage polarization through the STAT6-dependent signaling pathway
BMC Medicine (2022)
-
Gαi2-induced conductin/axin2 condensates inhibit Wnt/β-catenin signaling and suppress cancer growth
Nature Communications (2022)
-
Wnt signaling is boosted during intestinal regeneration by a CD44-positive feedback loop
Cell Death & Disease (2022)