Abstract
Defensins protect human barriers from commensal and pathogenic microorganisms. Human α-defensin 6 (HD-6) is produced exclusively by small intestinal Paneth cells but, in contrast to other antimicrobial peptides (AMPs) for HD-6, no direct antibacterial killing activity has been detected so far. Herein, we systematically tested how environmental factors, like pH and reducing conditions, affect antimicrobial activity of different defensins against anaerobic bacteria of the human intestinal microbiota. Remarkably, by mimicking the intestinal milieu we detected for the first time antibacterial activity of HD-6. Activity was observed against anaerobic gut commensals but not against some pathogenic strains. Antibiotic activity was attributable to the reduced peptide and independent of free cysteines or a conserved histidine residue. Furthermore, the oxidoreductase thioredoxin, which is also expressed in Paneth cells, is able to reduce a truncated physiological variant of HD-6. Ultrastructural analyses revealed that reduced HD-6 causes disintegration of cytoplasmic structures and alterations in the bacterial cell envelope, while maintaining extracellular net-like structures. We conclude that HD-6 is an antimicrobial peptide. Our data suggest two distinct antimicrobial mechanisms by one peptide: HD-6 kills specific microbes depending on the local environmental conditions, whereas known microbial trapping by extracellular net structures is independent of the reducing milieu.
Similar content being viewed by others
INTRODUCTION
The human intestine is one of the world’s most densely populated habitats, harboring ∼100 trillion organisms from which the majority are bacteria.1 Among them, facultative or strict anaerobes make up to 99% of the human gut bacteria that are dominated by Bacteroidetes and Firmicutes.2, 3 These vast numbers of microorganisms form a permanent threat to the host that is kept in check by an efficient intestinal defense system preventing microorganisms from translocating the epithelial barrier. One mechanism of human border control is employed by antimicrobial peptides (AMPs) that can be secreted by intestinal epithelia and help to keep the mucus layer overlaying gut epithelial cells sterile.4, 5 One of the most important groups of AMPs are the defensins, which are small, cationic peptide antibiotics.6, 7, 8 They protect the host against bacteria, fungi, and some viruses and shape the composition of the intestinal microbiota.9, 10, 11, 12
Paneth cells are secretory cells of the small intestine that are located in the bottom of the crypts of Lieberkühn. Several genetic defects in Paneth cells have been associated with ileal Crohn’s disease, an inflammatory bowel disease, thus suggesting an important role of this cell in the pathogenesis of ileal Crohn’s disease.13, 14, 15, 16, 17, 18 One major function of Paneth cells is the secretion of AMPs, mainly α-defensins 5 (HD-5) and 6 (HD-6).19, 20, 21 Moreover, diminished expression of HD-5 and HD-6 has been associated with ileal Crohn’s disease, and recently also with graft-versus-host disease.9, 22 For HD-5 this association could be easily understood as HD-5 has been shown to exhibit antibiotic function and to control the composition of the intestinal microbiota.9, 10, 23 In contrast, for HD-6, such clear experimental evidence has been lacking as the peptide had no detectable antimicrobial activity in vitro. Because of its high similarity and coexpression with HD-5, the lack of an obvious antimicrobial function was puzzling. Only recently, Chu et al.24 could show that HD-6 self-assembles into extracellular nanonets that entrap Salmonella enterica serovar Typhimurium in the intestine and thereby prevent translocation across the epithelial barrier.
AMPs have been mainly tested under standard conditions that do not necessarily reflect the conditions that occur in vivo. By simulating the reducing conditions as they occur in the intestine and other body locations,25 we could show that only under these conditions human β-defensin 1 (hBD-1) exhibits potent activity against several members of the human intestinal microbiota.26 In addition, reduction and activation of hBD-1 can be achieved enzymatically by the thioredoxin (TRX) system in vitro and in cell culture systems,26, 27 independent of the environment. As a reducing environment and an acidic pH occur naturally in the human gut, we tested systematically how these conditions influence antimicrobial activity of human α- and β-defensins. Instead of focusing on pathogenic bacteria as most previous analyses we investigated antimicrobial activity against (facultative) anaerobic bacteria residing as commensals in the human gut.
RESULTS
Environmental factors modulate antimicrobial activity of human defensins
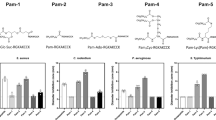
Environmental factors as pH and redox potential vary between different body sites. In a systematic analysis we compared how these factors influence antimicrobial activity of the most prominent human α- and β-defensins against (anaerobic) commensal gut bacteria. Therefore, we modified the radial diffusion assay26, 28 by applying acidic pH and/or reducing conditions. The reducing capability of the agarose gel was verified by addition of the redox indicator resazurin (Supplementary Figure 1a online) that confirmed a reducing milieu over incubation time (Supplementary Figure 1b).
As shown in Figure 1 for α-defensins and in Figure 2 for β-defensins, the effect of environmental conditions varies not only for different defensins but also for different bacteria. The influence seems specific for each individual defensin–bacterium pair and does not correlate with bacterial genus, cell wall structure (Gram status), or class of defensin. Whereas for Escherichia coli all tested defensins except HD-6 have their highest inhibiting activity under “standard conditions” (pH 7.4 and no reducing agent), for other bacteria the activity spectrum was more diverse. For instance, a reducing environment decreased antimicrobial activity of human neutrophil peptide 1–3 (HNP1–3) against E. coli, Bifidobacterium adolescentis, Bifidobacterium longum, and Streptococcus salivarius ssp thermophilus, whereas the same environment increased activity of HNP-4 against Bif. adolescentis, Lactobacillus acidophilus, and Bif. longum (Figure 1). Changing pH from 7.4 to 5.7 resulted in lower activity of hBD-2 against E. coli, L. acidophilus, and Lactobacillus fermentum, but for Bif. adolescentis the effect was not as obvious (Figure 2). Consequently, every defensin has distinct optimum conditions for inhibiting growth of different bacteria, and these conditions do not necessarily reflect the standard conditions on which most antimicrobial assays have been focused previously.
Systematic overview of how environmental conditions change α-defensin activity. 1 μg of α-defensins was tested against Escherichia coli, Bifidobacterium adolescentis Ni3,29c, Lactobacillus acidophilus, Bifidobacterium breve, Bacteroides vulgatus, Lactobacillus fermentum, Bifidobacterium longum, and Streptococcus salivarius ssp thermophilus under standard conditions (pH 7.4), reducing conditions (2 mM DTT), acidic conditions (pH 5.7), or a combination of both (pH 5.7+2 mM DTT). Diameter of inhibition zones was measured in radial diffusion assays to evaluate antimicrobial activity. A diameter of 2.5 mm (dotted line) is the diameter of the punched well/vehicle control and corresponds to no antimicrobial activity. All experiments were carried out at least three times; means with s.e.m. are shown. DTT, dithiothreitol; HD, human α-defensin; HNP, human neutrophil peptide.
Systematic overview of how environmental conditions change β-defensin activity. 1 μg of β-defensins was tested against Escherichia coli, Bifidobacterium adolescentis Ni3,29c, Lactobacillus acidophilus, Bifidobacterium breve, Bacteroides vulgatus, Lactobacillus fermentum, Bifidobacterium longum, and Streptococcus salivarius ssp thermophiles under standard conditions (pH 7.4), reducing conditions (2 mM DTT), acidic conditions (pH 5.7), or a combination of both (pH 5.7+2 mM DTT). Diameter of inhibition zones was measured in radial diffusion assays to evaluate antimicrobial activity. A diameter of 2.5 mm (dotted line) is the diameter of the punched well/vehicle control and corresponds to no antimicrobial activity. All experiments were carried out at least three times; means with s.e.m. are shown. DTT, dithiothreitol; hBD, human β-defensin.
Besides increased activity for hBD-1 and HNP-4 under reducing conditions against several bacteria, we detected for the first time antibiotic activity of the Paneth cell α-defensin HD-6 (Figure 1). The role of HD-6 has long been cryptic as this intestinal defensin did not show antimicrobial activity in standard killing assays, even though it represents the second most abundant AMP in Paneth cells.9 However, when testing anaerobic commensal bacteria we observed inhibitory activity of HD-6, especially under reducing conditions against Bif. adolescentis, L. acidophilus, and Bifidobacterium breve, as well as Bif. longum and S. thermophilus (Figure 1).
Reduction of HD-6 is a one-step reaction and reduced HD-6 is more hydrophobic
To ensure that the activation of HD-6 is indeed caused by a reducing milieu, we utilized the alternative reducing agent tris(2-carboxyethyl)phosphine instead of dithiothreitol (DTT) when analyzing Bif. adolescentis in radial diffusion assay. As a similar effect was observed (data not shown), we concluded that HD-6 exhibits antimicrobial activity in a reducing environment. As it is likely that the applied environment leads to incomplete or full chemical reduction of the disulfide bridges of HD-6, we tested this hypothesis by mass-spectrometric analysis. Oxidized HD-6 (oxHD-6) (expected: 3,706.54 m/z) was incubated with 2 mM DTT and free cysteines were subsequently alkylated by iodoacetamide, leading to an m/z shift of +58 per cysteine (+348 for six cysteine residues). We detected signals corresponding to the oxidized peptide (3,706.6 m/z) and to peptides with five (3,997.9 m/z) or six (4,055.0 m/z) reduced and alkylated cysteines (Figure 3a). In addition, we detected a nonidentified signal at 3,974.4 m/z that occurred during the alkylation process. Accordingly, under the applied assay conditions a fraction of HD-6 is completely reduced whereas another fraction remains in its oxidized state. These results were confirmed by reversed-phase high-performance liquid chromatography (HPLC) analysis (Figure 3b) in which incubation with DTT led to a shift from the oxidized peptide to the reduced, more hydrophobic form, revealed by an increased retention time. No signals were detected for intermediate forms with only one or two reduced disulfide bridges, thereby supporting the mass-spectrometry results and suggesting an all-or-nothing mechanism. Furthermore, comparable results were obtained by nano-HPLC coupled to a quadrupole-time-of-flight mass spectrometer. After incubation of oxHD-6 with 2 mM DTT (Figure 3d), the chromatogram contained two peaks with mass-to-charge-ratio (m/z) signals indicating 3-, 4-, 5-, and 6-fold protonated ions of either oxidized HD-6 (neutral mass M=3,705.4961 Da) or completely reduced HD-6 (neutral mass M=3,711.5430 Da). Again, no mass signals of intermediate redox forms were detectable. Extending the incubation time to 3 h or increasing DTT concentration to 10 mM remarkably enhanced HD-6 reduction, but the oxidized form of the peptide could still be detected (data not shown). Hence, under our antimicrobial assay conditions HD-6 seems to contain a mixture of fully oxidized and fully reduced HD-6.
HD-6 is reduced in a one-step reduction without intermediate, partly reduced forms. (a) oxHD-6 was incubated with or without 2 mM DTT, alkylated with iodoacetamide, and analyzed by matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). Mass spectra contain mass-to-charge ratios of singly protonated ions: 3,706.62 m/z for oxidized HD-6 and 4,054.95 m/z for completely reduced and sixfold alkylated HD-6. Each alkylated, formerly free cysteine results in an increased m/z of +57. (b) oxHD-6 or (c) ΔoxHD-6 was incubated with or without 2 mM DTT and analyzed by reversed-phase high-performance liquid chromatography (RP-HPLC) to investigate hydrophobicity and intermediate forms of HD-6. (d) oxHD-6 was incubated with 2 mM DTT and analyzed by nano-HPLC coupled to a quadrupole-time-of-flight mass spectrometer equipped with an electrospray-ionization source. The mass spectra of the peaks contain mass-to-charge ratios (m/z) that correspond to 3-, 4-, 5-, and 6-fold protonated ions of either oxidized HD-6 (neutral mass M=3,705.4961 Da) or completely reduced HD-6 (neutral mass M=3,711.5430 Da). The neutral mass difference of ∼6 reflects the difference of six hydrogen atoms between the two redox forms. The signal at 1,221.9906 m/z is derived from the reference mass that is present in all analyses. For each subfigure, one representative experiment out of three independent experiments is shown. DTT, dithiothreitol; HD-6, α-defensin 6; IAA, iodoacetamide; oxHD-6, oxidized HD-6; redHD-6, reduced HD-6; UV, ultraviolet.
Although for HD-6 the major form has been isolated as a 32-amino acid peptide, some amino-terminally processed forms have also been identified from ileal neobladder urine and from ileal mucosa extract.29 We therefore tested reduction of the amino-terminally truncated peptide ΔoxHD-6 lacking the N-terminal alanine and phenylalanine. In contrast to the 32-amino acid peptide, the truncated peptide having 30 amino acids was completely reduced by 2 mM DTT (Figure 3c and Supplementary Figure 2). However, the truncated ΔoxHD-6 acted comparable in the radial diffusion assay (Supplementary Figure 3), ruling out the possibility that these two amino acids are essential for the observed activity.
The reduced HD-6 peptide inhibits growth of Bif. adolescentis
In a next step we investigated whether the antimicrobial activity of HD-6 is attributable to a reduced form of the peptide. We therefore compared oxHD-6 and reduced HD-6 (ΔredHD-6) in their ability to inhibit growth of Bif. adolescentis. Whereas oxHD-6 inhibited bacterial growth only weakly in a liquid broth assay, ΔredHD-6 caused concentration-dependent growth inhibition of Bif. adolescentis over 48 h (Figure 4a). However, when including the reducing agent DTT (Figure 4b) in the assay, both peptides prevented growth of Bif. adolescentis in a concentration-dependent manner, supporting the idea that a reduced form of HD-6 exhibits direct antimicrobial activity. As most investigations on HD-6 were performed under standard conditions and with (opportunistic) pathogenic bacteria, we investigated whether a reducing environment also modulates HD-6 function against a selection of pathogenic microbes. However, we did not observe substantial antimicrobial activity against E. coli, Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, Salmonella typhimurium, or the yeast Candida albicans under any tested conditions (Figure 4c). Taken together, these results demonstrate that antimicrobial activity of HD-6 seems to be specific for a subset of commensal bacteria and dependent on reducing conditions.
Comparison of antimicrobial activity of different HD-6 forms. (a) Antimicrobial activity of oxidized and reduced HD-6 (ΔredHD-6) was compared in a turbidity liquid culture assay against Bifidobacterium adolescentis Ni3,29c. Different concentrations of peptides were incubated with bacteria and change in optical density (OD600) was measured after 48 h in the absence (a) or presence (b) of 2 mM DTT. (c) 2 μg of oxHD-6, ΔredHD-6, or oxHD-6 under reducing conditions (oxHD-6+2 mM DTT) were tested in a radial diffusion assay against (opportunistic) pathogenic bacteria and the yeast Candida albicans. A diameter of 2.5 mm (dotted line) is the diameter of punched well/vehicle control and corresponds to no antimicrobial activity. All experiments were carried out at least three times; means with s.e.m. are shown. DTT, dithiothreitol; HD-6, α-defensin 6; n.d., not determined; oxHD-6, oxidized HD-6; redHD-6, reduced HD-6.
Extracellular net-like structures are preserved under reducing conditions
Oxidized HD-6 prevents S. typhimurium from translocating the intestinal mucosa by forming molecular peptide nanonets.24 To investigate how peptide reduction affects net formation, we performed scanning electron microscopy analyses under reducing conditions. When incubating Gram-negative S. typhimurium with ΔredHD-6 (Figure 5a, top row) we observed extracellular structures that resembled those previously described for the oxidized peptide.24 Consequently, reduction of the three disulfide bridges does not per se affect the ability to form extracellular peptide nets. Mesh-like structures were also observed when using the Gram-positive commensal gut bacterium Bif. adolescentis (Figure 5a, middle row), but these structures were yet different in their appearance. The extracellular structures seemed less organized and included rather compact material. As reduced HD-6 is able to kill Bifidobacterium but not Salmonella, it is possible that additional bacterial debris is associated with HD-6 structures. This hypothesis was strengthened by utilizing protein A-coated beads instead of bacteria (Figure 5a, bottom row). After incubating the beads with ΔredHD-6 we observed net-like structures that were similar to the structures that have been observed for S. typhimurium. Thus, reduced HD-6 retains the ability to form ordered extracellular associations that form independently of the presence of bacteria but may also attract bacterial debris and other particles.
Reduced HD-6 forms extracellular net-like structures while causing intracellular alterations. (a) Gram-negative S. typhimurium (top row), Gram-positive Bif. adolescentis Ni3,29c (middle row), or protein A-coated beads (bottom row) were treated with 0.01% acetic acid (control) or with 10 μg ml−1 of ΔredHD-6 in the presence of 2 mM DTT and investigated by scanning electron microscopy. Magnification bars are shown in representative images. (b) Bif. adolescentis Ni3,29c were treated with 0.01% acetic acid (control) or with 200 μg ml−1 of ΔredHD-6 in the presence of 2 mM DTT and investigated by transmission electron microscopy. Magnification bar=0.2 μm. DTT, dithiothreitol; HD-6, α-defensin 6; redHD-6, reduced HD-6.
To investigate whether HD-6 treatment results in visible intracellular alterations in Bifidobacteria we next performed transmission electron microscopy (Figure 5b). Incubation of Bif. adolescentis with ΔredHD-6 resulted in disintegration of stainable intracellular structures. The bacterial envelope was evidently detached from the bacterial cytoplasm and extracellular aggregations at the cell envelope were detectable. Nevertheless, no pore formation or blebbing, which are common mechanisms for AMPs,30, 31 were observed during transmission electron microscopy or by scanning electron microscopy. Hence, by a so far unknown mechanism reduced HD-6 initiates intracellular processes, leading to alterations in bacterial cytoplasm and cell envelope structure, and finally causing bacterial death.
Antimicrobial activity of reduced HD-6 is not dependent on His25 or free cysteine residues
As a histidine residue at position 27 (His27) is critical to prevent bacterial invasion by HD-6,24 we investigated whether histidine residue (at position 25 in our reduced peptide, see above) is essential for antimicrobial activity of reduced HD-6. Unexpectedly, replacing His by Trp did not abolish activity, rather antimicrobial activity was slightly increased as shown by liquid broth antimicrobial assay (Figure 6a) and colony-forming unit (CFU) counts (Figure 6b). Similarly, replacing all six cysteine residues by α-aminobutyric acid (Abu), a steric analog of the amino acid cysteine, led to even better antimicrobial activity. Both peptide variants were still able to form extracellular net-like structures between Bif. adolescentis and S. typhimurium (Figure 6c). As revealed by transmission electron microscopy of Bif. adolescentis, incubation with ΔredHD-6(H25W) or ΔredHD-6(Abu) led to similar ultrastructural alterations of the bacterium as observed with reduced HD-6 (Figure 5b). A loss of intracellular integrity is observable, along with a detachment of the plasma membrane (Figure 6d). Consequently, these experiments suggest that neither the histidine residue nor free sulfhydryl groups are essential for antimicrobial activity of the reduced HD-6 peptide; rather, a linear structure of the peptide seems to be crucial.
Antimicrobial activity of reduced HD-6 is independent of His25 and free cysteines. Different concentrations of oxidized HD-6 (oxHD-6) and reduced HD-6 (ΔredHD-6) as well as variants in which His25 is replaced by tryptophan (ΔredHD-6(H25W)) or in which all cysteine residues have been replaced by α-aminobutyric acid (ΔredHD-6(Abu)) were tested in a turbidity liquid culture assay against Bif. adolescentis Ni3,29c. Peptides were incubated with bacteria and change in optical density (OD600nm) was measured after 48 h (a) or aliquots were plated on agar plates and colony-forming units (CFUs) were calculated the next day (b). Please note that apparent better growth of bacteria incubated with higher concentrations of oxidized HD-6 is caused by light-scattering aggregations and could not be detected with CFU counting as shown in b. Experiments were carried out three times, means with s.e.m. are shown. (c) Scanning electron microscopy (SEM) of Bif. adolescentis ATCC 15703 and S. typhimurium of ΔredHD-6(H25W) and ΔredHD-6(Abu) (each 10 μg ml−1) in the presence of 2 mM DTT. (d) Transmission electron microscopy (TEM) of ΔredHD-6(H25W) and ΔredHD-6(Abu) (each 200 μg ml−1) after incubation with Bif. adolescentis Ni3,29c in the presence of 2 mM DTT. Magnification bars in the panels indicate a length of 0.2 μm. DTT, dithiothreitol; HD-6, α-defensin 6.
Physiological reduction of oxidized HD-6
As the oxidoreductase TRX is expressed in Paneth cells,32 can be secreted,33 and is able to reduce hBD-1 in vitro26 and in the supernatant of a cell culture system,27 we investigated whether the TRX system is also able to catalyze reduction of HD-6. As shown in Figure 7a, the TRX system consisting of NADPH, thioredoxin-reductase (TRX-Red), and TRX catalyzes partial reduction of oxHD-6 (upper row). Increasing the concentration of TRX to 6 μM or prolonging the incubation time to 60 min was not sufficient to completely reduce oxHD-6 (data not shown). However, the truncated form ΔoxHD-6 can be completely reduced by only 30 min of incubation and 3 μM of TRX (Figure 7a, bottom row). Protein disulfide isomerase, glutathione, glutaredoxin, or sodium sulfide (Na2S), the sodium salt of hydrogen sulfide produced by intestinal microbiota,34 did not lead to any reduction of HD-6 (Supplementary Figure 4).
Physiological aspects of antimicrobial activity by HD-6. (a) Oxidized HD-6 (top row) or truncated ΔoxHD-6 (bottom row) were incubated with rat thioredoxin reductase, NADPH, and with or without 3 μM thioredoxin (TRX). Enzymatic conversion from oxidized HD-6 into the reduced form was investigated by RP-HPLC analysis. Representative experiments of three independent experiments are shown. (b) Schematic model describing a potential mechanism of HD-6 activity. HD-6 might be secreted as an oxidized peptide by Paneth cells into the crypt, trap bacteria by formation of nanonets that further diffuse toward the lumen. The reducing milieu might reduce HD-6 that eventually kills bacteria. For more details please see text. (c) Bif. adolescentis Ni3,29c bacteria were incubated in the absence (untreated) or presence of oxidized HD-6 for 30 min without anaerobic environment, followed by 90 min of incubation in an anaerobic environment in the presence or absence of DTT. Aliquots were plated on agar plates and colony-forming units were calculated the next day. Experiments were carried out at least three times; means with s.e.m. are shown. DTT, dithiothreitol; HD-6, α-defensin 6; oxHD-6, oxidized HD-6; redHD-6, reduced HD-6; RP-HPLC, reversed-phase high-performance liquid chromatography; UV, ultraviolet.
In addition to reduction by the TRX system we also suggest an alternative activation mechanism of HD-6. We hypothesize that HD-6 is secreted as an oxidized peptide at the bottom of the small intestinal crypt into an aerobic milieu and can be reduced spontaneously (nonenzymatically) when reaching the reducing environment closer to the lumen (Figure 7b). To mimic such a scenario, we incubated Bif. adolescentis with 60 μg ml−1 of oxHD-6, a concentration being much lower than 15–100 mg ml−1 that have been proposed for α-defensins in intestinal crypts.35 After preincubation under aerobic conditions for 30 min, we applied a reducing environment for 90 min by addition of DTT. As shown in Figure 7c, for Bifidobacteria solely incubated with oxidized HD-6, a minor decrease in CFU numbers was observed. In contrast, application of reducing conditions after 30 min led to an evident decrease in CFUs. Although the in vivo proof of such a scenario remains to be shown, we hypothesize that such a two-step mechanism might expand the functional role of HD-6 in small intestinal crypts.
DISCUSSION
AMPs have been mainly investigated under standard laboratory conditions, including a defined medium, a pH of 7.4, and the presence of oxygen and well-classified, mainly pathogenic, bacteria or fungi. However, the long road through the human intestine includes areas with a pH of ∼5.7–6.4 in the duodenum, 7.3–7.7 in the ileum, and ∼5.6–5.7 in the ascending and transverse colon, then increasing to 6.6–6.8 in the rectum.36 In addition, the redox potential decreases from ∼−150 mV in the ileum down to −200 to −300 mV in the colon.36 In the present systematic analysis we found that these environmental conditions influence the antimicrobial activity of human defensins against several (facultative) anaerobic bacteria. It seems that optimal activity conditions for each defensin were different for individual bacteria and might be related to the physiological presence of bacteria in the niches described above. Remarkably, Bacteroides vulgatus (Figures 1 and 2) is comparably resistant under most conditions against defensin treatment. It may be speculated that there is a causal link between increased defensin resistance and the high prevalence of Bacteroides genus in the stool of healthy humans.37
Oxidized HD-6 has been shown to form nanonets to entrap Gram-negative Salmonella.24 Here we found that reduced HD-6 does not only form net-like structures between Salmonella but also between Gram-positive Bif. adolescentis. Hence, HD-6 might protect the intestinal epithelium not only against pathogenic Salmonella, but also against members of the commensal microbiota. However, whereas Bifidobacteria were killed by reduced HD-6, this was not the case for S. typhimurium. Consequently, formation of extracellular net structures seems to be independent of the antimicrobial activity of HD-6. This is supported by our finding that killing of Bif. adolescentis does not require a conserved histidine residue. Similarly, replacing cysteine residues in the reduced HD-6 by a steric analog lacking free sulfhydryl groups did not abolish antimicrobial activity. It might thus be the case that a linear form of the peptide is able to interact with a so far unknown molecular target or structural component that might be inaccessible to the oxHD-6 peptide.
After applying reducing conditions to oxHD-6 it occurs as a mixture of fully oxidized and fully reduced peptide (Figure 3). When directly comparing the antimicrobial activity of this mixture with pure prereduced ΔredHD-6 the peptide mixture has slightly higher activity (Figure 4b) in a liquid broth assay. Hence, it seems that an initial interaction or trapping by the oxidized peptide is beneficial for subsequent killing of Bifidobacteria, once the peptide is reduced.
After incubation of Bif. adolescentis with reduced HD-6, a detachment of the bacterial envelope was observed and extracellular aggregations at the bacterial cell envelope were visible. The cell envelope, which is an essential part of the bacterium, is a common target for antibiotics and AMPs.38, 39, 40 It is thus possible that reduced HD-6 interferes with enzymes that are crucial for cell wall biosynthesis, or that reduced HD-6 sequesters substrates being essential for cell wall build-up. Nevertheless, although radial diffusion assay and turbidity broth assay do not differentiate between bacteriostatic and bactericidal activity, results from electron microscopy clearly support a bactericidal mechanism.
In search of a physiological mediator we tested different redox systems and low-molecular-weight thiols for their ability to reduce HD-6. The only system being able to reduce HD-6 was the TRX system that can also reduce hBD-1 and HD-5.26, 27, 41 While the presumably prevailing form of HD-6 was only partly reduced, the N-terminally truncated form ΔoxHD-6 was efficiently and completely reduced. Structural investigations by Chu et al.24 provide evidence that the amino terminal Ala1 and Phe2 are involved in dimer–dimer association with adjacent cysteine residues. It may thus be speculated that this interaction as well as the resulting steric hindrance impedes reduction of the involved cysteine residues, and might explain why reduction of the truncated form lacking Ala1 and Phe2 is facilitated. However, these two amino acids seem not to be crucial for the observed antimicrobial activity as both peptides show similar results in the radial diffusion assay. To assess the role of both peptide forms in vivo, further studies are needed to quantify their relative amount in the human intestine.
Because of their architecture and in contrast to the lumen, small intestinal crypts are characterized by an aerobic environment as demonstrated for colonic crypts.42, 43 The Paneth cells at the bottom of small intestinal crypts secrete HD-6 most likely as an oxidized peptide that is able to trap bacteria by forming extracellular nanonets.24 The peptide will then diffuse toward the lumen where a reducing milieu is prevailing. We thus hypothesize that, in addition to enzymatic reduction by TRX, such an environment might activate HD-6, thereby protecting the small intestinal crypts by direct killing of commensal microorganisms. This Retiarius-like mechanism suggests that net formation might rather entrap bacteria in small intestinal crypts, whereas bactericidal activity of HD-6 would occur at locations closer to the intestinal lumen. Thus, our data propose two distinct antimicrobial mechanisms by one peptide. Microbes are trapped by forming extracellular nanonets24 and, as demonstrated here, this net formation process occurs independent of the environmental redox situation. In contrast, the second antimicrobial mechanism, however, is depending on the environment: only after activation by a reducing luminal milieu or by TRX, HD-6 exhibits antimicrobial activity. Together, these two mechanisms might broaden the host defense repertoire and increase the antimicrobial effectiveness of epithelial barrier function. This may well be relevant in clinical conditions like Crohn’s disease where expression of the AMP HD6 is diminished.
METHODS
Microbial strains. Bacterial strains Bifidobacterium adolescentis Ni3,29c, Bifidobacterium breve PZ 1343, Bifidobacterium longum DSM 20219T, Lactobacillus acidophilus PZ 1138, Lactobacillus fermentum PZ 1162, and Streptococcus salivarius ssp thermophilus (S. thermophilus) DSM20617 were obtained from Ardeypharm (Herdecke, Germany) and Bacteroides vulgatus DSM1447 was obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Candida albicans ATCC 10231, and Escherichia faecalis ATCC 29212 were provided by the Department for Laboratory Medicine at Robert Bosch Hospital (Stuttgart, Germany). Salmonella enterica serovar Typhimurium (Salmonella typhimurium) ATCC 14028 was kindly provided by Andreas Bäumler (UC Davis, Davis, CA), and Bifidobacterium adolescentis ATCC 15703 was kindly provided by David Mills (UC Davis).
Peptides. For all experiments, synthetic oxidized peptides HNP-4, HD-5, HD-6, hBD-1, hBD-2, and hBD-3 (Peptide Institute, Osaka, Japan), as well as natural human HNP1–3 (Hycult Biotech, Uden, The Netherlands) were dissolved in 0.01% acetic acid at a concentration of 1 μg μl−1. Reduced HD-6 (ΔredHD-6), ΔredHD-6(Abu), ΔredHD-6(H25W), and ΔoxHD-6 were synthesized by EMC Microcollections GmbH (Tuebingen, Germany).
Antimicrobial radial diffusion assay. Antimicrobial radial diffusion assay was performed as described previously.26, 28 Briefly, logarithmic phase bacteria were washed and diluted to optical density (OD600 nm)=0.1; 150 μl was used for killing assays. Incubation was carried out in 10 mM sodium phosphate (pH 7.4 or 5.7) containing 0.3 mg ml−1 of trypticase soy broth (TSB) powder and 1% (w/v) low EEO-agarose (Applichem, Darmstadt, Germany) with 0–2 mM DTT (Sigma Aldrich, Steinheim, Germany) or 0–2 mM tris(2-carboxyethyl)phosphine (Carl Roth, Karlsruhe, Germany). The redox indicator resazurin (Sigma Aldrich) was added to the gel (2 μg ml−1), which did not influence bacterial growth. Peptides were pipetted into punched wells and allowed to diffuse into the gel. An overlay gel was poured onto the plates after 3 h, and after up to 48 h the diameter of inhibition zones was measured. Solvent (0.01% acetic acid) was used as vehicle control and did not show any inhibition zones reaching beyond the 2.5 mm diameter of the punched well. All experiments were carried out at least three times.
Turbidity broth assay. Briefly, Bif. adolescentis Ni3,29c was incubated overnight, centrifuged, and washed twice with 10 mM sodium phosphate buffer containing 1% (w/v) TSB broth. Approximately 5 × 105 CFU per ml bacteria were mixed with indicated peptide concentrations (1.25–128 μg ml−1) in a final volume of 100 μl in 10 mM sodium phosphate buffer containing 1% (w/v) TSB broth and incubated for 2 h at 37 °C in an anaerobic jar. 2 mM DTT was included into the assay where indicated. After incubation, 100 μl of 6% TSB (w/v) was added and absorbance was measured at 600 nm (EnSpire, Perkin-Elmer, Santa Clara, CA). Bacterial growth was monitored after 24 and 48 h and change in absorbance was plotted against peptide concentration. In addition, for some experiments, after an initial 2 h of incubation, 10 μl of bacterial suspension was mixed with TSB medium and several dilutions were plated on Columbia Blood agar plates to determine CFU. For Figure 7c, incubation was carried out at 37 °C without anaerobic atmosphere for 30 min, followed by addition of DTT and incubation in an anaerobic jar for another 90 min. Bacterial suspensions were mixed with TSB medium and two dilutions were plated on Columbia Blood agar plates to determine CFU. All experiments were carried out at least three times.
HPLC analysis. Oxidized HD-6 was incubated with or without 2 mM DTT in 10 mM sodium phosphate buffer, pH 7.4, for 30 min at 37 °C. HPLC analysis was carried out with an Agilent 1200 series system (Agilent, Santa Clara, CA) and a Vydac 218 TP-C18 Column (250 × 4.6 mm, 5 μm, Grace, Hesperia, CA). Gradient increased from 15% B to 45% B in 30 min (solvent A=water+0.18% (v/v) trifluoroacetic acid; solvent B=acetonitrile+0.15% (v/v) trifluoroacetic acid) at 25 °C and 0.8 ml min−1.
Matrix-assisted laser desorption/ionization mass spectrometry. Oxidized HD-6 was incubated with or without 2 mM DTT in 10 mM sodium phosphate buffer, pH 7.4, for 30 min at 37 °C, followed by alkylation with 20 mM iodoacetamide for 30 min at 25 °C and co-crystallized with α-cyano-4-hydroxy cinnamic acid. Matrix-assisted laser desorption/ionization mass spectrometry was carried out at an ultraflex TOF/TOF machine (Bruker, Bremen, Germany).
Analysis by nano-HPLC and electrospray mass spectrometry. 2 μg ΔoxHD-6 and oxHD-6 were incubated with 2 mM DTT at pH 7.4 for 30 min at 37 °C. Subsequently, samples were acidified with 0.1% formic acid and 10% acetonitrile was added. 0.5 μl was injected for analysis on a 6540 UHD Q-TOF LC/MS system (Agilent), a quadrupole-time-of-flight mass spectrometer equipped with an electrospray-ionization source. Chromatographic separation was carried out by nano-HPLC (ChipCube, Agilent) with a reversed-phase column using an Agilent HPLC ProtID-Chip-43 with a 40 nl enrichment column and a 75 μl × 43 mm analytical column made of ZORBAX 300SB-C18 5 μm material. Analytes were separated by a gradient of acetonitrile in 0.1% formic acid. Mass spectrometric analysis was done in the MS mode from 100 to –1,700 m/z with positive ion polarity. Data were analyzed by Agilent MassHunter Quantitative Analysis B 06.00 software.
In vitro reduction assay. Reduction assays were performed as previously described,26 analogous to Holmgren et al.44 Briefly, 7.5 μM synthetic oxHD-6 or ΔoxHD-6 was incubated with 0.8 mM NADPH (Biomol, Hamburg, Germany), rat TRX reductase (200 nM, IMCO, Stockholm, Sweden), and 3.0 μM human TRX (Sigma Aldrich) or 3.0 μM bovine protein disulfide isomerase (Sigma Aldrich) in 0.1 M potassium phosphate buffer containing 2 mM EDTA at pH 7.0 for 30 min at 37 °C. For the oxidoreductase glutaredoxin, a glutathione-based system was used. 5 mM reduced glutathione (Carl Roth) was incubated in 100 mM sodium phosphate buffer with 2 mM EDTA and 3 μM active glutaredoxin (Abcam, Cambridge, UK) or an equivalent volume of buffer. Furthermore, 50 μM Na2S (VWR BDH Prolabo, Dublin, Ireland) in 0.1 M potassium phosphate buffer containing 2 mM EDTA, pH 7.0, was incubated with 7.5 μM oxidized HD-6. Incubation mixtures were acidified with trifluoroacetic acid, mixed with HPLC solvent, and analyzed by HPLC as described above.
Scanning electron microscopy of bacteria. Scanning electron microscopy was based on protocols described by Chu et al.24 Briefly, ∼1 × 107 CFUs of Bif. adolescentis ATCC 15703 or Bif. adolescentis Ni3,29c, ∼4 × 106 CFUs of S. typhimurium, or protein A-coated beads (Spherotech, Lake Forest, IL) were incubated for 30 min at room temperature in 10 mM sodium phosphate buffer (pH 7.4) with 10 μg ml−1 synthetic reduced ΔHD-6 or its variants in the presence of 2 mM DTT. Addition of DTT did not lead to visible changes in bacterial structures (data not shown). As a control, incubation of reduced ΔHD-6 with 2 mM DTT in sodium phosphate buffer ensured that the peptide retained its completely reduced status for at least 2 h. Bacteria and beads were then centrifuged, washed with 50 mM Tris-maleate buffer, pH 6.4+150 mM NaCl, and fixed in Karnovsky’s reagent or 2.5% glutaraldehyde overnight. Samples were dehydrated to 100% ethanol and critical-point dried from CO2 and examined either at 15 kV accelerating voltage in a Hitachi S-800 field emission scanning electron microscope (Tokio, Japan) or at 20 kV accelerating voltage in a Philips XL-30 scanning electron microscope (Amsterdam, The Netherlands).
Transmission electron microscopy of bacteria. Approximately 2 × 108 CFU/ml of Bif. adolescentis Ni3,29c were incubated for 2 h at 37 °C in 10 mM sodium phosphate buffer (pH 7.4) containing 0.3 mg ml−1 of TSB powder with 200 μg ml−1 synthetic ΔredHD-6 in the presence of 2 mM DTT. As control, bacteria were incubated with an equal volume of solvent (0.01% acetic acid). Bacteria were centrifuged, fixed in prewarmed Karnovsky’s fixative for 1 h at room temperature, and stored at 4 °C for 24 h. After centrifugation, the sediment was embedded in 3.5% agarose at 37 °C, coagulated at room temperature, cut in small blocks, and fixed again in Karnovsky’s solution for at least 1 h. Postfixation was based on 1.0% osmium tetroxide containing 1.5% K-ferrocyanide in 0.1 M cacodylate buffer for 2 h. After embedding in glycide ether, the blocks were cut using an Ultracut microtome (Reichert, Wien, Austria). Ultra-thin sections (30 nm) were mounted on copper grids and analyzed using a Zeiss LIBRA 120 transmission electron microscope (Carl Zeiss, Jena, Germany) operating at 120 kV.
References
Ley, R.E., Peterson, D.A. & Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837–848 (2006).
Eckburg, P.B . et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005).
Lozupone, C.A ., Stombaugh, J.I ., Gordon, J.I ., Jansson, J.K . & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Johansson, M.E.V. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 105, 15064–15069 (2008).
Meyer-Hoffert, U. et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut 57, 764–835 (2008).
Lehrer, R.I ., Ganz, T. & Selsted, M.E. Defensins: endogenous antibiotic peptides of animal cells. Cell 64, 229–230 (1991).
Martin, E., Ganz, T. & Lehrer, R.I. Defensins and other endogenous peptide antibiotics of vertebrates. J. Leukoc. Biol. 58, 128–136 (1995).
Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 (2003).
Wehkamp, J. et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc. Natl. Acad. Sci. USA 102, 18129–18134 (2005).
Salzman, N.H . et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 11, 76–83 (2010).
Bevins, C. & Salzman, N. The potter’s wheel: the host’s role in sculpting its microbiota. Cell. Mol. Life Sci. 68, 3675–3760 (2011).
Ostaff, M.J ., Stange, E.F . & Wehkamp, J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med. 5, 1–19 (2013).
Kaser, A. & Blumberg, R.S. Autophagy, microbial sensing, endoplasmic reticulum stress, and epithelial function in inflammatory bowel disease. Gastroenterology 140, 1738–1747 (2011).
Kaser, A. & Blumberg, R.S. ATG16L1 Crohn’s disease risk stresses the endoplasmic reticulum of Paneth cells. Gut 63, 1038–1039 (2013).
Adolph, T.E . et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–322 (2008).
VanDussen, K.L . et al. Genetic variants synthesize to produce paneth cell phenotypes that define subtypes of Crohn’s disease. Gastroenterology 146, 200–209 (2014).
Thachil, E. et al. Abnormal activation of autophagy-induced crinophagy in Paneth cells from patients with Crohn’s disease. Gastroenterology 142, 1097–1099.e4 (2012).
Bevins, C.L . & Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368 (2011).
Jones, D.E . & Bevins, C.L. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 315, 187–192 (1993).
Wehkamp, J. & Stange, E.F. Paneth’s disease. J. Crohns Colitis 4, 523–531 (2010).
Eriguchi, Y. et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood 120, 223–231 (2012).
Porter, E.M ., van, Dam, E., Valore, E.V . & Ganz, T. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect. Immun. 65, 2396–2401 (1997).
Chu, H. et al. Human α-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science 337, 477–481 (2012).
Nagy, E. Anaerobic infections: update on treatment considerations. Drugs 70, 841–858 (2010).
Schroeder, B.O . et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 469, 419–423 (2011).
Jaeger, S.U . et al. Cell-mediated reduction of human β-defensin 1: a major role for mucosal thioredoxin. Mucosal Immunol. 6, 1179–1190 (2013).
Lehrer, R.I ., Rosenman, M., Harwig, S.S ., Jackson, R. & Eisenhauer, P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods 137, 167–173 (1991).
Porter, E.M . et al. Isolation of human intestinal defensins from ileal neobladder urine. FEBS Lett. 434, 272–276 (1998).
Mukherjee, S. et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 505, 103–107 (2014).
Sass, V. et al. Human beta-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect. Immun. 78, 2793–2800 (2010).
Takaishi, S. et al. Growth promoting effect of thioredoxin on intestinal epithelial cells. Dig. Dis. Sci. 48, 379–464 (2003).
Rubartelli, A., Bajetto, A., Allavena, G., Wollman, E. & Sitia, R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J. Biol. Chem. 267, 24161–24164 (1992).
Blachier, F. et al. Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids 39, 335–347 (2010).
Ayabe, T. et al. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1, 113–118 (2000).
Wilson, M. Microbial Inhabitants of Humans - Their Ecology and Role in Health and Disease, Cambridge University Press, University College London, (2005).
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Jordan, S., Hutchings, M.I . & Mascher, T. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol. Rev. 32, 107–146 (2008).
Schneider, T. et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science (New York, NY) 328, 1168–1240 (2010).
Peschel, A. & Sahl, H.-G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4, 529–536 (2006).
Zhang, Y., Cougnon, F.B.L., Wanniarachchi, Y.A ., Hayden, J.A . & Nolan, E.M. Reduction of human defensin 5 affords a high-affinity zinc-chelating peptide. ACS Chem. Biol. 8, 1907–1911 (2013).
Kelly, C.J . et al. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol. 6, 1110–1118 (2013).
Pédron, T. et al. A crypt-specific core microbiota resides in the mouse colon. MBio 3, e00116–12 (2012).
Holmgren, A. Enzymatic reduction-oxidation of protein disulfides by thioredoxin. Methods Enzymol. 107, 295–300 (1984).
Acknowledgements
We thank Michelle Katajew, Marion Strauβ, and Kathleen Siegel for excellent technical assistance, Birgit Fehrenbacher (University Hospital Tuebingen) and Patricia Kysar (UC Davis) for performing electron microscopy, Dr Matthias Flötenmeyer (MPI Tuebingen) for assistance with scanning electron microscopy, and Dr Christoph Pöhlmann (Robert Bosch Hospital, Stuttgart) for providing and helping with pathogenic bacterial strains. Furthermore, we specially thank Professor Charles L. Bevins (UC Davis) for providing laboratory space and equipment and for critical data discussions. This study was supported by the European Union (ERC Starting Grant to J.W.) and the Robert Bosch Foundation, Stuttgart, Germany. B.O.S. was supported by DACED Zukunftspreis (Ferring Arzneimittel GmbH, Germany) and a Boehringer Ingelheim Fonds Travel Grant. This work is also supported by Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
Author Contributions
B.O.S. performed antimicrobial activity assays, HPLC analyses, and MALDI-MS, reduction assays, was involved in sample preparation for electron microscopy analyses, designed and evaluated experiments, generated figures, and wrote the manuscript. D.E. performed antimicrobial activity assays, HPLC analyses, and MALDI-MS. J.C.P. performed and evaluated Q-TOF-MS analyses and was involved in generating figures and writing of the manuscript. P.A.C. assisted in and performed scanning microscopy analyses. R.K. tested antimicrobial activity against pathogenic microorganisms, J.B. performed scanning microscopy experiments and M.S. was in charge of transmission electron microscopy. E.F.S. was involved in data discussions and writing of the manuscript. J.W. was involved in data discussions, evaluation of experiments, writing of the manuscript, and design of the study. All authors were involved in data discussions and the final version of the manuscript.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Schroeder, B., Ehmann, D., Precht, J. et al. Paneth cell α-defensin 6 (HD-6) is an antimicrobial peptide. Mucosal Immunol 8, 661–671 (2015). https://doi.org/10.1038/mi.2014.100
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2014.100
This article is cited by
-
Resistance is futile? Mucosal immune mechanisms in the context of microbial ecology and evolution
Mucosal Immunology (2022)
-
Therapeutic Potential of Antimicrobial Peptides for Wound Healing
International Journal of Peptide Research and Therapeutics (2022)
-
Potent bactericidal activity of reduced cryptdin-4 derived from its hydrophobicity and mediated by bacterial membrane disruption
Amino Acids (2022)
-
HPV infection alters vaginal microbiome through down-regulating host mucosal innate peptides used by Lactobacilli as amino acid sources
Nature Communications (2022)
-
Pathogen-specific antimicrobials engineered de novo through membrane-protein biomimicry
Nature Biomedical Engineering (2021)