Abstract
Follicular dendritic cell secreted protein (FDC-SP) is a secreted peptide predominantly expressed in mucosal tissues. We previously reported that FDC-SP transgenic mice have altered B-cell responses to systemic immunization; however, the role of FDC-SP in mucosal immunity is unknown. Here, we report that FDC-SP functions in regulating immunoglobulin A production. FDC-SP transgenic mice show decreased IgA levels in serum, saliva, and bronchoalveolar lavage fluid. Reciprocally, FDC-SP-deficient mice show significantly increased IgA levels in serum and intestinal lavage, associated with accumulation of IgA+ cells in blood, bone marrow, Peyer’s patches, and lymph nodes. FDC-SP-deficient mice generated higher titers of antigen-specific IgA but normal IgG1 responses upon immunization. Purified FDC-SP transgenic B cells generated decreased IgA responses to transforming growth factor β (TGFβ)+interleukin 5 (IL5) stimulation. Consistent with a direct effect of FDC-SP on B cells, recombinant FDC-SP suppressed B-cell IgA production in vitro. Six- to 14-month-old FDC-SP-deficient mice show IgA deposition in kidney glomeruli, which was associated with proteinuria and pathology consistent with mild IgA nephropathy (IgAN). Our results demonstrate a novel biological activity of FDC-SP in controlling B-cell IgA production and identify FDC-SP-deficient mice as a novel mouse model of IgAN.
Similar content being viewed by others
INTRODUCTION
The follicular dendritic cell secreted protein (FDC-SP) gene encodes an 85 amino-acid secreted protein with a unique expression pattern. We previously reported that human FDC-SP is expressed within human tonsils and lymph nodes, but not spleen, gut tissues, or blood leukocytes.1 Within tonsil, FDC-SP is expressed in germinal centers, FDCs, and also within tonsillar crypts.1 FDC-SP expression is inducible by bacterial products such as lipopolysaccharide (LPS) or killed Staphylococcus aureus Cowan, or inflammatory stimuli such as tumor necrosis factor α.1 While FDC-SP was identified via its expression in FDCs, it was subsequently reported to be expressed in salivary gland and periodontal ligament,2 head and neck cancers,3 and ovarian/breast cancers.4, 5, 6 The fdc-sp/c4orf7 gene is located within a cluster of genes encoding small secreted proteins present in secretions including saliva and milk.7 FDC-SP protein is present in saliva8 and FDC-SP mRNA was recently identified by genome-wide profiling as a highly selective marker for human saliva.9 Transgenic mice constitutively expressing mouse FDC-SP in all lymphoid tissues were found to generate reduced serum IgG2b and IgG3 responses but normal IgM and IgG1 responses after immunization;10 however, the function of FDC-SP in mucosal immunity remains unknown.
Generation of mucosal IgA antibody in the gastrointestinal tract and other mucosal sites likely share a number of common mechanisms at the level of induction of IgA isotype switch and homing of mucosal-primed B cells.11 A number of cytokines are implicated in IgA production including transforming growth factor β (TGFβ)12 and the tumor necrosis factor ligand family members B cell activating factor and a proliferation-inducing ligand.13 IgA responses also appear to be driven by commensal microorganisms, via both innate mechanisms such as microbial induction of IgA switch factors14 and specific recognition of microbial antigens.15, 16, 17 B cells primed for IgA isotype switch at mucosal inductive sites such as NALT, BALT, and GALT are programmed with specific homing receptors to promote return to mucosal sites after recirculation.11, 18, 19 IgA-producing plasma cells can be found in many mucosal areas, including tonsil, salivary glands, nasal passage, and gut lamina propria.20, 21, 22
Abnormal systemic IgA production can lead to IgA deposition in the kidney, resulting in IgA nephropathy (IgAN).23, 24 Mucosal induction sites are proposed to play an important role in generating systemic IgA and IgA-related pathology;25, 26 however, the underlying mechanisms are not understood. Clinically, IgAN frequently presents as an acute episode of kidney dysfunction (detected by hematuria and proteinuria) often closely associated with infection such as tonsillitis, pharyngitis, or “flu”,27 suggesting that excessive mucosal IgA responses triggered by pathogens may have a role. In some parts of Asia, tonsillectomy is routinely performed as a treatment for IgAN patients;28 however, the efficacy of this approach has not been validated in a large controlled trial.26 Clearly much remains to be learned about the regionally specialized mechanisms controlling IgA generation and their impact on systemic IgA levels.
In this study, we probe the function of FDC-SP in mucosal immunity using FDC-deficient and transgenic mice, focusing on oral and respiratory IgA responses. Strikingly, we find that FDC-SP deficiency and overexpression have reciprocal effects on IgA, with FDC-SP-deficient mice showing significantly elevated salivary and serum IgA, while transgenic mice show reduced levels. We further show that FDC-SP-deficient mice generate increased Ag-specific IgA responses upon immunization, show systemic accumulation of IgA+ cells, and develop IgAN-like kidney pathology. These results provide the first evidence that FDC-SP can modulate mucosal immune responses and suggest that this peptide may directly act on B cells to regulate production of IgA.
RESULTS
FDC-SP transgenic mice show suppression of IgA production
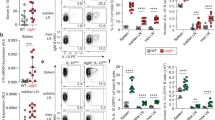
We have previously generated and characterized FDC-SP transgenic mice in terms of their Ag-specific serum IgM and IgG responses generated after immunization.10 Given the accumulating data suggesting FDC-SP may function at mucosal surfaces we assessed IgA antibody levels in serum, saliva, and bronchoalveolar lavage fluid (BALF) from these mice. We found that FDC-SP transgenic serum had significantly reduced levels of IgA compared with controls, whereas IgG1 and IgM antibody levels were not significantly different (Figure 1a). Saliva also showed significantly reduced IgA levels (Figure 1b), while, in contrast, salivary IgG1 levels were significantly increased. BALF derived from FDC-SP transgenic mice also showed abnormal antibody levels, with reduced IgA but elevated IgG1 (Figure 1c). These results suggest that overexpression of FDC-SP can suppress IgA production and deregulate IgG1 production in oral and respiratory fluids.
Follicular dendritic cell secreted protein (FDC-SP) transgenic (TG) mice show reduced IgA levels. Serum (a), saliva (b), and bronchoalveolar lavage fluid (c; BALF) were collected from 8- to 12-week-old FDC-SP transgenic or control mice. Levels of the indicated immunoglobulin isotypes were assessed using specific ELISAs. Box and whisker graphs represent the upper and lower quartile ranges (box), the median (line across the box) and maximum and minimum measured values (whiskers) from a cohort of eight mice per genotype. Results are representative of two independent experiments with different mouse cohorts. Statistical significance was determined by T-test (*P<0.05; **P<0.01).
FDC-SP-deficient mice show enhanced production of IgA and other Ig isotypes
To determine the impact of physiologically expressed FDC-SP on antibody production, FDC-SP-deficient mice were generated by gene targeting (Supplementary Figure S1 online). FDC-SP-deficient mice did not show any gross abnormalities in lymphoid tissues and B-cell development appears to be normal as determined by flow cytometry (Supplementary Figure S2A). The major lymphoid and myeloid cell subsets were present at normal frequencies (Supplementary Figure S2B). Serum, saliva, and BALF were collected from young adult mice and levels of antibody isotypes were assessed (Figure 2). FDC-SP-deficient mice showed significantly elevated levels of all Ig isotypes examined in serum, while saliva and BALF showed increased levels of IgA and IgG1. We further examined generation of antigen-specific IgA upon immunization with 4-hydroxy-3-nitrophenyl-ovalbumin+LPS and found that FDC-SP-deficient mice generated significantly higher titers of anti-NP IgA than controls, whereas anti-NP IgG1 titers were not significantly different (Figure 3). We assessed the frequencies of IgA+ B cells in various tissues of FDC-SP-deficient mice and found striking, variable increases in IgA+ cells in blood, cervical lymph nodes, and bone marrow, whereas IgG1+ cells were not significantly increased (Figure 4a,b). These IgA+ cells were largely B220+ and CD138lo, consistent with an abnormal accumulation of IgA-switched pre-plasma cells; however, increased populations of B220lo, CD138+ and intracellular IgA+ plasma cells could also be observed in bone marrow (Figure 4c). However, the proportion of IgA+ cells bearing an intracellular-IgAhi/CD138+ phenotype was not increased in FDC-SP-deficient mice (Figure 4d), suggesting that differentiation of IgA-switched cells into plasma cells is not affected. Together, these data indicate that FDC-SP deficiency leads to deregulated generation of IgA+ B cells and has a reciprocal effect on IgA levels relative to FDC-SP transgenic mice.
Follicular dendritic cell secreted protein (FDC-SP)-deficient mice show increased levels of IgA, IgG1, and IgM in serum, saliva, and bronchoalveolar lavage fluid (BALF). Serum (a), saliva (b), and BALF (c) were collected from 8- to 12-week-old FDC-SP-deficient or control mice. Levels of the indicated immunoglobulin isotypes were assessed using specific ELISAs. Box and whisker graphs represent the upper and lower quartile ranges (box), the median (line across the box) and maximum and minimum measured values (whiskers) from a cohort of eight mice per genotype. Results are representative of three independent experiments with different mouse cohorts. Statistical significance was determined by T-test (*P<0.05; **P<0.01).
Antigen-specific antibody responses in follicular dendritic cell secreted protein (FDC-SP)-deficient mice. Mice were immunized intraperitoneally with 4-hydroxy-3-nitrophenyl-ovalbumin (NP-OVA), alum, and lipopolysaccharide (LPS) and serum (a) and bronchoalveolar lavage fluid (b; ALF) harvested at day 14. Titers of NP-binding IgA or IgG1 antibodies were determined using NP-BSA coated ELISA plates. Statistical significance was determined by T-test (*P<0.05). Similar results were seen in two independent experiments.
Follicular dendritic cell secreted protein (FDC-SP)-deficient mice show increased frequencies of IgA+ cells. Cells were isolated from the indicated tissues and analyzed by flow cytometry to determine the frequencies of IgA-positive cells. (a) Representative dot plots indicate gating of IgA+ B cells. The IgA/IgG1 plots shown are gated on live B220+CD4−CD8− lymphocytes. (b) Percent IgA+ or IgG1+ cells in 10- to 12-week-old FDC-SP-deficient or control mice. Box and whisker graphs represent the upper and lower quartile ranges (box), the median (line across the box) and maximum and minimum measured values (whiskers) from 12 to 16 mice per genotype. Statistical significance was determined by T-test (*P<0.05; **P<0.01, ***P<0.001). (c) Accumulated IgA+ cells in FDC-SP knockout (KO) mice are B220hi. Top panels show staining of bone marrow. Bottom panels show cervical lymph node. (d) Accumulated IgA+ cells in FDC-SP KO mouse bone marrow consist of both B220hi/CD138lo and B220lo/CD138hi populations. Bottom flow-cytometry plots show intracellular IgA staining of bone marrow to identify plasma cells (PCs). Graphs on the right show iIgAloCD138− or iIgAhiCD138+ cells as a percentage of total lymphocytes or iIgAhiCD138+ PCs as a percent of total IgA+ cells. Results are averaged from eight mice per genotype. WT, wild type.
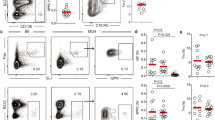
Although FDC-SP expression has not been prominently observed in the gastrointestinal tract,1, 2, 3 it is possible that swallowed salivary peptides (or fragments thereof) may reach the gut, or influence the gut through other indirect mechanisms of communication between mucosal sites. We examined IgA levels present in intestinal lavage and found that they were significantly increased, whereas IgM levels were normal (Figure 5a). Flow-cytometry analysis of mesenteric lymph nodes revealed normal frequencies of germinal center B cells and marginally elevated frequencies of IgA+ B cells (Figure 5b,c). Peyer’s patch B cells showed significantly elevated frequencies of IgA B cells (Figure 5c), with increases apparent in both plasma cells and non-plasma cells (Figure 5d). These results indicate that dysregulation of IgA in FDC-SP-deficient mice extends to gut-associated mucosal sites.
Intestinal IgA production in follicular dendritic cell secreted protein (FDC-SP)-deficient mice. (a) IgM or IgA antibodies present in gut lavage fluid were measured by ELISA (N=8 mice per genotype). Significance was determined by T-test (* denotes P<0.05). (b) Cells were isolated from the indicated tissues and analyzed by flow cytometry to determine the frequencies of GL7+Fas+ germinal center (GC) B cells (eight mice per genotype). (c) Surface IgA+ or IgG1+ cells in 10- to 12-week-old FDC-SP-deficient or control mesenteric lymph node (mLN) or Peyer’s patch were determined by flow cytometry. Left panels show representative staining profiles. Graphs on the right show percent IgA+ or IgG1+ cells from 8 to 12 mice per genotype. Significance was determined by T-test (* denotes P<0.05). (d) Intracellular IgA staining of Peyer’s patch to identify plasma cells (PCs). Left panels show representative intracellular IgA/CD138 staining profiles. Graphs on the right show iIgAloCD138− or iIgAhiCD138+ cells as a percentage of total lymphocytes or iIgAhiCD138+ PCs as a percent of total IgA+ cells. Results are averaged from eight mice per genotype. WT, wild type; KO, knockout.
FDC-SP can regulate IgA production in vitro
To determine whether FDC-SP can directly regulate B-cell production of IgA, we utilized established protocols to induce IgA production in vitro.29 Purified IgA-negative B cells isolated from control or FDC-SP transgenic mice were isolated, cultured with or without TGFβ, and then assessed for generation of IgA+ cells and secreted IgA (Figure 6). It was found that FDC-SP transgenic B cells produced significantly fewer IgA+ cells over the 5-day culture period (Figure 6a) and secreted significantly less IgA into culture supernatants (Figure 6b) compared with control B cells. In contrast, secreted IgM levels were not significantly different (Figure 6b, right panel). Using supernatants of L cell transfectants containing secreted FDC-SP, we determined whether addition of FDC-SP could affect IgA production. It was found that addition of FDC-SP supernatant significantly decreased IgA+ cells and IgA secretion compared with control supernatants, whereas IgM levels were not affected (Figure 6c). Together, these results indicate that FDC-SP can act directly on B cells to influence IgA production.
Follicular dendritic cell secreted protein (FDC-SP) can directly inhibit generation of IgA responses by purified IgA-negative B cells. (a) IgA-negative B cells isolated from spleens of FDC-SP transgenic (TG) or control mice were cultured in the presence of lipopolysaccharide (LPS)+IL5 or LPS+IL5+TGFβ to induce isotype switch to IgA. After 5 days, cells and supernatants were harvested. The frequency of IgA+ cells was determined by flow cytometry. Graph at right shows the average and standard error of results obtained from four independently assayed mice in a single experiment and are representative of three experiments. (b) Levels of secreted IgA (left panel) or IgM (right panel) were determined by ELISA analysis of culture supernatants. (c) Splenic B cells from wild-type (WT) C57BL6 mice were cultured with LPS+TGFβ+IL5 plus 10% supernatant from FDC-SP-expressing L cells or control L cells. Percent IgA+ or secreted IgA and IgM levels were assessed at day 5. Results show the average and standard error of replicate wells and are representative of three independent experiments. IL5, interleukin 5; TGFβ, transforming growth factor β.
FDC-SP knockout mice develop nephropathy with age
Chronic elevation of IgA levels is associated with kidney dysfunction in the disease IgA nephropathy (IgAN). To determine whether chronically elevated IgA levels in FDC-SP-deficient mice may impact on kidney function, we examined FDC-SP-deficient mice at 6–14 months of age. In aged mice, IgA levels remained elevated, whereas IgG1 levels normalized relative to a matched control cohort (Figure 7a). We examined kidney sections by light microscopy and found that FDC-SP glomeruli show variable mild mesangial cell proliferation (Figure 7b, top panels). Staining for IgA, IgG, and complement C3 revealed immune complex deposition in glomeruli of FDC-SP-deficient mice, but not in control mice (Figure 7b, middle panels). Examination by electron microscopy revealed electron dense deposits in mesangial extracellular matrix, consistent with mesangial immune complex deposition (Figure 7b, bottom panels). Urinalysis indicated variable hematuria, increased 24-h protein excretion, and slight elevation in serum creatinine in 8- to 14-month-old FDC-SP-deficient mice (Figure 7c), consistent with development of chronic kidney dysfunction. Interestingly, IgA levels were most consistently elevated in male FDC-SP-deficient mice and only male mice showed significantly increased protein excretion (Supplementary Figure S3). These data indicate that FDC-SP deficiency leads to chronic elevation of IgA, deposition of IgA in kidney glomeruli and kidney dysfunction with a male bias, similar to human IgAN.
Follicular dendritic cell secreted protein (FDC-SP)-deficient mice develop nephropathy associated with IgA deposition in kidney glomeruli. (a) Serum, saliva, and bronchoalveolar lavage fluid (BALF) were collected from mice at 6–14 months of age and serum IgA and IgG1 levels were determined. Note IgA levels remain elevated but, unlike young mice, IgG1 levels were not significantly different. (b) Kidney pathology analyses. Formalin-fixed sections were assessed by hematoxylin eosin (H&E) staining and imaged under × 600 magnification (top panels). Kidney cryosections were collected, stained with anti-IgA, anti-IgG, or anti-C3 antibodies and imaged under × 1,000 magnification (middle panels). Glutaraldehyde-fixed sections were examined under × 10,000 magnification by electron microscopy (EM) (bottom panels). Relative magnification is indicated. Examples of electron-dense mesangial immune complex-type deposits in FDC-SP-deficient kidneys are indicated by white arrows. Results are representative of four FDC-SP knockout (KO) mice examined by immunofluorescence and light microscopy and two FDC-SP KO mice examined by EM. (c) Urine and serum analyses. 24-h urine collections were taken from 6- to 8-month-old FDC-SP-deficient or control mice and total protein excretion was measured (top panel). Presence of red blood cells in urine samples was determined by urinalysis dipstick test (middle panel). Serum creatinine levels were determined in a cohort of 14-month-old mice (bottom panel). WT, wild type; PC, plasma cell.
DISCUSSION
This is the first report identifying a function of FDC-SP in mucosal immunity. Our results demonstrate that FDC-SP can directly regulate IgA production and that the absence of FDC-SP leads to deregulation of IgA. The restricted expression of FDC-SP within cervical lymph node, salivary gland, periodontal ligament, and oral-associated lymphoid tissue suggests that FDC-SP provides region-specific control of IgA homeostasis. We have previously found that FDC-SP expression can be induced by microbial products and inflammatory stimuli, suggesting that it may serve as an endogenous brake on IgA responses induced by pathogenic or commensal microorganisms in oral/respiratory mucosa.
FDC-SP transgenic mice show strongly inhibited IgA levels but relatively normal IgG and IgM levels, consistent with a relatively selective effect on IgA. Young adult FDC-SP-deficient mice were found to have elevated IgM, IgG, and IgA levels, possibly suggesting a global deregulation of natural antibody production. However, by 6 months of age IgM and IgG levels were found to be similar to control mice, while IgA levels remained elevated. Immunization studies indicated that FDC-SP knockout (KO) mice generated significantly higher titers of antigen-specific IgA, while generation of Ag-specific IgM and IgG were relatively normal. While we cannot rule out other immunological defects in these mice, the current data strongly indicate that a selective effect of FDC-SP on generation of IgA-expressing B cells is one of the activities of this peptide.
Elevated numbers of IgA+ B cells were found in cervical lymph nodes, blood, and bone marrow of FDC-SP-deficient mice. The presence of significant numbers of IgA+ cells in blood is intriguing and suggests potential spill-over of IgA-switched B cells from mucosal sites. Marginally elevated frequencies of IgA+ B cells were also observed in gut-associated lymphoid tissues, significantly impacting on gut IgA levels. Since FDC-SP is mainly expressed at oral and respiratory sites, these results may indicate that swallowed salivary FDC-SP can influence the gut. Alternatively, FDC-SP may indirectly affect gut IgA production via modulation of microbiota or via other systemic effects. IgA+ cells did not accumulate in spleen, consistent with lack of FDC-SP expression in spleen and selective homing of mucosal-primed B cells to mucosal sites.11
Most of the accumulated IgA+ cells did not appear to be plasma cells, suggesting either that abnormal numbers of IgA+ B cells are continually generated, or that there is an abnormal accumulation of long-lived IgA+ B cells. While increased frequencies of intracellular IgAhi/CD138+ plasma cells were found in bone marrow and Peyer’s patches, the proportion of IgA+ cells expressing a plasma cell phenotype was not significantly higher in FDC-SP-deficient mice. This result argues against a role for FDC-SP in promoting differentiation of IgA-switched cells into plasma cells. In vitro studies with purified B cells supported a direct effect of FDC-SP on induction of both surface IgA+ and IgA-secreting cells, perhaps acting at the level of isotype switch. Since FDC-SP was found to bind selectively to B cells,1 the simplest hypothesis is that an FDC-SP-binding receptor can transduce signals inhibiting IgA isotype switch. We previously also found evidence that FDC-SP could influence migration of activated B cells,1 thus another possibility is that FDC-SP deficiency could impact tissue homing of IgA+ B cells. We hypothesize that the FDC-SP gene gives rise to multiple processed peptides with multiple immune-modulatory functions, analogous to other immune-modulatory secreted peptides expressed at mucosal surfaces and in saliva,30, 31, 32 but has a non-redundant function impacting IgA production.
FDC-SP-deficient mice have a phenotype with several similarities to human IgAN, including chronic elevation of IgA, deposition of IgA immune complexes in kidney, and kidney dysfunction. Relatively mild kidney pathology is a feature of many cases of IgAN, and the disease is often undetected until routine urinalysis reveals proteinuria or hematuria.23, 24 Human IgAN is more frequent in males, consistent with findings in FDC-SP KO mice. Interestingly, human IgAN is frequently associated with respiratory infections that may provoke episodes of hyper-IgA production leading to acute kidney dysfunction.27, 33 IgAN patients have been shown to have abnormal numbers of IgA+ cells in tonsils34 and abnormal response to mucosal immune challenge.35 Tonsillectomy is a routine intervention for IgAN in Japan, intended to dampen mucosal IgA production;28 however, the efficacy of this approach has not been proven by a large-scale trial. Given the evidence that abnormal mucosal immune responses are associated with IgAN, it will be interesting to examine responses to oral or respiratory pathogens in FDC-SP-deficient mice and determine whether they exacerbate IgAN-like pathology.
A few other mouse models of IgAN have been described, including the ddY mouse and B cell activating factor transgenic mice.36 B cell activating factor transgenic mice produce a more severe, acute form of IgAN with very large elevation of IgA and IgG and significant kidney pathology affect both males and females.25, 37 The ddY mouse exhibits spontaneous glomerular IgA deposition and provides a multigenic disease model.38, 39 The original ddY model was not consistently associated with elevated IgA levels, but a variant of the strain with hyper-IgA and accelerated disease progression was recently described.40 On the basis of the results here, FDC-SP-deficient mice provide a novel single-gene deficiency model on a defined genetic background with potential utility for studying a biological process with relevance to IgAN, namely mucosal induction of IgA responses. It should be noted that, as is the case with other mouse models, FDC-SP-deficient mice cannot recapitulate all aspects of human IgAN pathology. For example, mouse IgA is not structurally equivalent to human IgA1 and thus is unlikely to precisely mimic altered glycosylation patterns seen in this molecule that are thought to contribute to IgAN pathogenesis.41
In summary, this study provides the first evidence supporting a function of FDC-SP in mucosal immunity. Genetic overexpression and deficiency of FDC-SP have reciprocal effects on IgA levels and this peptide can directly inhibit B-cell IgA production in vitro, together providing strong evidence implicating FDC-SP as a new regulator of IgA synthesis. While further studies will be required to delineate the molecular and cellular mechanisms by which FDC-SP controls IgA responses, the FDC-SP-deficient mouse has clear potential as a model of IgAN.
METHODS
FDC-SP-deficient and transgenic mice. FDC-SP transgenic CD1 mice were generated as described.10 FDC-SP-deficient mice were generated as illustrated in Supplementary Figure S1. Heterozygous mice bearing the targeted collapsed FDC-SP locus were generated by crossing with Cre transgenic and homozygous FDC-SP-deficient mice were generated by subsequent back-crossing to C57BL6 for seven generations. Mice were genotyped using DNA extracted from ear punches, using the following primers:
Wild-type allele (430 bp product):
GGGATAAAGTGATAAAAACGAATAGCCA and ACGGAAATCCAGAAGATGCAAGCCT
KO allele (522 bp product):
GGGATAAAGTGATAAAAACGAATAGCCA and GGGGCCACCAAAGAACGGAGCCGGTT
All experimental animals were housed at the Central Animal Care Facility (University of Manitoba, Winnipeg, Manitoba, Canada) in compliance with the guidelines established by the Canadian Council on Animal Care. CD1 and C57BL6 control mice were bred separately within same animal facility as the FDC-SP transgenic and FDC-SP-deficient mice.
Flow-cytometry analyses. Single-cell suspensions were generated from the indicated tissues and were pre-incubated with 2.4G2 antibody to block Fc receptors. The indicated conjugated antibodies were added from the following panel: V500-labeled anti-CD4, PE anti-CD5, FITC anti-CD11b, FITC anti-CD21, PE anti-CD23, PerCP anti-CD45R/B220, FITC anti-CD45/B220, PE anti-CD43, PE anti-Gr1, APC anti-CD183, APC anti-IgM and FITC-anti-IgG1 (all from BD Biosciences, San Jose, CA), Pacific Blue anti-CD8, AlexaFluor647 anti-mouse CD4, and PE anti-mouse IgA (eBioscience, San Diego, CA). For intracellular staining, cells were first stained with PerCP-anti-B220, APC anti-CD138, and LIVE/DEAD Aqua fixable dead cell stain (Invitrogen, Carlsbad, CA), then fixed and permeabilized using CytofixCytoperm solution (BD Biosciences) and finally intracellularly stained with PE-anti-IgA. Stained cells were washed and acquired on a FACS Canto II flow cytometer (BD Biosciences). Data were analyzed using the FlowJo software (TreeStar, Ashland, OR).
Immunization and antibody measurements. Serum, saliva, BALF, and gut lavage42 fluids were collected from 10- to 14-week-old FDC-SP-deficient mice, FDC-SP transgenic mice and strain, age and sex-matched controls. Where indicated mice were immunized intraperitoneally with 4-hydroxy-3-nitrophenyl-ovalbumin, alum, and LPS. For ELISAs, 96-well assay plates were coated overnight at 4 °C with capture antibodies or antigen diluted in carbonate coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3, 0.05% NaN3, pH 9.6). Total antibody levels were determined by coating with anti-mouse IgM, IgG1, or IgA (Jackson ImmunoResearch Laboratories, West Grove, PA) or NP-specific antibody levels were determined by coating plates with NP20-BSA (Biosearch Technologies, Petaluma, CA). Detection was carried out using biotinylated anti-mouse IgM, IgG1, or IgA antibodies (Southern Biotechnology, Birmingham, AL) followed by streptavidin alkaline phosphatase.
B-cell isolation and cultures. Mouse splenocytes were collected and B cells were purified by negative selection with the CD43 MicroBeads and MACS columns (Miltenyi Biotech, Bergisch Gladbach, Germany). Purified B cells were incubated in dishes coated with goat anti-mouse IgA (4 μg ml−1) for 70 min at 4 °C, resulting in >95% depletion of sIgA+ cells. IgA-depleted B cells were then washed and resuspended in complete medium (Opti-MEM containing penicillin-streptomycin, 2-mercaptoethanol and 10% FBS). A total of 4 × 105 cells per well were cultured in flat-bottomed, 96-well tissue-culture plates in a volume of 100 μl complete medium containing 10 μg ml−1 LPS (Escherichia coli 0127:B8; Sigma Chemical, St Louis, MO), 100 U ml−1 murine interleukin 5 (IL5) (R&D Systems, Minneapolis, MN), and 1 ng ml−1 TGFβ1 (R&D Systems). After 5 days of culture, supernatants were harvested for ELISA analysis and cells for flow cytometry. FDC-SP containing L cell supernatants were generated from stable transfectants as described.10 Serum-free supernatants were prepared by extensively washing and culturing confluent L cells in medium containing 0.5% BSA for overnight. Supernatants were harvested and used immediately or aliquoted and stored frozen at –80 °C.
Urine and kidney analyses. Kidneys were harvested and either embedded in O.C.T. compound (Tissue Tek, Tokyo, Japan) and snap frozen using liquid nitrogen for cryosectioning, 10% formalin fixed for light microscopy or 2% glutaraldehyde fixed for electron microscopy. Cryosections were cut at 8 μm using a cryostat and placed onto slides (Fisherbrand Superfrost/Plus, Waltham, MA). Frozen sections were fixed for 15–20 min using ice-cold acetone, blocked with 10% normal goat serum for 1 h and stained with either FITC anti-IgA or anti-IgG (Southern Biotechnology). Complement deposition was detected by staining with anti-C3 antibody (Abcam, Cambridge, UK, #ab11862) followed by Alexa 488-goat anti-rat IgG (Abcam #ab150157). After mounting, sections were imaged using a confocal microscope (Ultraview LCI, Perkin-Elmer, Waltham, MA). For pathology analyses, formalin-fixed kidneys were paraffin embedded, sectioned and stained with hematoxylin and eosin stain; glutaraldehyde-fixed kidney samples were embedded in resin and ultrasectioned for examination with electron microscopy. For 24 h urine protein assay, individual mice were put in a metabolic cage with water but no food for 24 h. Collected urine was clarified by centrifugation at 3,000 r.p.m. and protein measured using Bradford protein assay (Bio-Rad, Hercules, CA). Total protein was determined by multiplying protein concentration by volume and was independently measured three times per animal.
References
Marshall, A.J. et al. FDC-SP, a novel secreted protein expressed by follicular dendritic cells. J. Immunol. 169, 2381–2389 (2002).
Nakamura, S. et al. Identification of genes preferentially expressed in periodontal ligament: specific expression of a novel secreted protein, FDC-SP. Biochem. Biophys. Res. Commun. 338, 1197–1203 (2005).
Li, F. et al. Identification and characterization of a novel nasopharyngeal carcinoma-associated peptide: NAP-1. J. Transl. Med. 2, 10 (2004).
Zucchi, I. et al. Gene expression profiles of epithelial cells microscopically isolated from a breast-invasive ductal carcinoma and a nodal metastasis. Proc. Natl. Acad. Sci. USA 101, 18147–18152 (2004).
Wu, M. et al. Development and characterization of a novel method for the analysis of gene expression patterns in lymphatic endothelial cells derived from primary breast tissues. J. Cancer. Res. Clin. Oncol. 136, 863–872 (2010).
Wang, C. et al. C4orf7 contributes to ovarian cancer metastasis by promoting cancer cell migration and invasion. Oncol. Rep. 24, 933–939 (2010).
Kawasaki, K, Lafont, A.G. & Sire, J.Y. The evolution of milk casein genes from tooth genes before the origin of mammals. Mol. Biol. Evol. 28, 2053–2061 (2011).
Guo, T. et al. Characterization of the human salivary proteome by capillary isoelectric focusing/nanoreversed-phase liquid chromatography coupled with ESI-tandem MS. J. Proteome Res. 5, 1469–1478 (2006).
Park, S.M. et al. Genome-wide mRNA profiling and multiplex quantitative RT-PCR for forensic body fluid identification. Forensic Sci. Int. Genet. 7, 143–150 (2012).
Al-Alwan, M. et al. Follicular dendritic cell secreted protein (FDC-SP) regulates germinal center and antibody responses. J. Immunol. 178, 7859–7867 (2007).
Macpherson, A.J., McCoy, K.D., Johansen, F.E. & Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008).
Cazac, B.B. & Roes, J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 13, 443–451 (2000).
Castigli, E. et al. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 201, 35–39 (2005).
He, B. et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 (2007).
Smith, D.J., King, W.F. & Taubman, M.A. Salivary IgA antibody to oral streptococcal antigens in predentate infants. Oral Microbiol. Immunol. 5, 57–62 (1990).
Percival, R.S., Marsh, P.D. & Challacombe, S.J. Age-related changes in salivary antibodies to commensal oral and gut biota. Oral Microbiol. Immunol. 12, 57–63 (1997).
Macpherson, A.J. et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226 (2000).
Bienenstock, J & McDermott, M.R. Bronchus- and nasal-associated lymphoid tissues. Immunol. Rev. 206, 22–31 (2005).
McDermott, M.R. & Bienenstock, J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 122, 1892–1898 (1979).
Brandtzaeg, P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann. NY Acad. Sci. 1098, 288–311 (2007).
Fritz, J.H. et al. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature 481, 199–203 (2011).
Kiyono, H & Fukuyama, S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat. Rev. Immunol. 4, 699–710 (2004).
Mestecky, J. et al. IgA nephropathy: molecular mechanisms of the disease. Annu. Rev. Pathol. 8, 217–240 (2013).
Wyatt, R.J. & Julian, B.A. IgA nephropathy. N. Engl. J. Med. 368, 2402–2414 (2013).
McCarthy, D.D. et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J. Clin. Invest. 121, 3991–4002 (2011).
Suzuki, Y. et al. Pathological role of tonsillar B cells in IgA nephropathy. Clin. Dev. Immunol. 2011, 639074 (2011).
Emancipator, S.N. Immunoregulatory factors in the pathogenesis of IgA nephropathy. Kidney Int. 38, 1216–1229 (1990).
Xie, Y. et al. The efficacy of tonsillectomy on long-term renal survival in patients with IgA nephropathy. Kidney Int. 63, 1861–1867 (2003).
Sonoda, E. et al. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J. Exp. Med. 170, 1415–1420 (1989).
Mathison, R.D., Davison, J.S., Befus, A.D. & Gingerich, D.A. Salivary gland derived peptides as a new class of anti-inflammatory agents: review of preclinical pharmacology of C-terminal peptides of SMR1 protein. J. Inflamm. (Lond) 7, 49 (2010).
Fabian, T.K., Hermann, P., Beck, A., Fejerdy, P. & Fabian, G. Salivary defense proteins: their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 13, 4295–4320 (2012).
Choi, K.Y., Chow, L.N. & Mookherjee, N. Cationic host defence peptides: multifaceted role in immune modulation and inflammation. J. Innate Immun. 4, 361–370 (2012).
Pouria, S & Barratt, J. Secondary IgA nephropathy. Semin. Nephrol. 28, 27–37 (2008).
Bene, M.C., Hurault De Ligny, B., Kessler, M. & Faure, G.C. Confirmation of tonsillar anomalies in IgA nephropathy: a multicenter study. Nephron 58, 425–428 (1991).
de Fijter, J.W. et al. Deficient IgA1 immune response to nasal cholera toxin subunit B in primary IgA nephropathy. Kidney Int. 50, 952–961 (1996).
Emancipator, S.N. Prospects and perspectives on IgA nephropathy from animal models. Contrib. Nephrol. 169, 126–152 (2011).
McCarthy, D.D., Chiu, S, Gao, Y, Summers-deLuca, L.E. & Gommerman, J.L. BAFF induces a hyper-IgA syndrome in the intestinal lamina propria concomitant with IgA deposition in the kidney independent of LIGHT. Cell Immunol. 241, 85–94 (2006).
Imai, H. et al. Spontaneous glomerular IgA deposition in ddY mice: an animal model of IgA nephritis. Kidney Int. 27, 756–761 (1985).
Tomino, Y. IgA nephropathy: lessons from an animal model, the ddY mouse. J. Nephrol. 21, 463–467 (2008).
Okazaki, K. et al. Development of a model of early-onset IgA nephropathy. J. Am. Soc. Nephrol. 23, 1364–1374 (2012).
Tanaka, M, Seki, G, Someya, T, Nagata, M & Fujita, T. Aberrantly glycosylated IgA1 as a factor in the pathogenesis of IgA nephropathy. Clin. Dev. Immunol. 2011, 470803 (2011).
Elson, C.O., Ealding, W & Lefkowitz, J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J. Immunol. Methods 67, 101–108 (1984).
Acknowledgements
We thank Dr Monther Al-Alwan for initial characterization of FDC-SP transgenic mice and Terry Lees and Terry Germscheid for excellent management of animal colonies. We are grateful to Drs Ted Yun, Sambasiva Rao, Evangelia Hatzis, and Jingbo Zhang for helpful discussions; Martin Scott and Svetlana Shulga-Morskaya for help with gene targeting; and Baher Nashed for help with back-crossing FDC-SP-deficient mice. Funding was provided by the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Sen Hou and Aaron Marshall hold a patent related to the subject matter of this study.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Hou, S., Landego, I., Jayachandran, N. et al. Follicular dendritic cell secreted protein FDC-SP controls IgA production. Mucosal Immunol 7, 948–957 (2014). https://doi.org/10.1038/mi.2013.113
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2013.113
This article is cited by
-
Follicular dendritic cell-secreted protein gene expression is upregulated and spread in nifedipine-induced gingival overgrowth
Odontology (2020)
-
The regulation of gut mucosal IgA B-cell responses: recent developments
Mucosal Immunology (2017)
-
Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses
Mucosal Immunology (2017)