Abstract
Human immunodeficiency virus (HIV) and Simian immunodeficiency virus (SIV) disease progression is associated with multifocal damage to the gastrointestinal tract epithelial barrier that correlates with microbial translocation and persistent pathological immune activation, but the underlying mechanisms remain unclear. Investigating alterations in mucosal immunity during SIV infection, we found that damage to the colonic epithelial barrier was associated with loss of multiple lineages of interleukin (IL)-17-producing lymphocytes, cells that microarray analysis showed expressed genes important for enterocyte homeostasis, including IL-22. IL-22-producing lymphocytes were also lost after SIV infection. Potentially explaining coordinate loss of these distinct populations, we also observed loss of CD103+ dendritic cells (DCs) after SIV infection, which associated with the loss of IL-17- and IL-22-producing lymphocytes. CD103+ DCs expressed genes associated with promotion of IL-17/IL-22+ cells, and coculture of CD103+ DCs and naïve T cells led to increased IL17A and RORc expression in differentiating T cells. These results reveal complex interactions between mucosal immune cell subsets providing potential mechanistic insights into mechanisms of mucosal immune dysregulation during HIV/SIV infection, and offer hints for development of novel therapeutic strategies to address this aspect of AIDS virus pathogenesis.
Similar content being viewed by others
Introduction
During progressive HIV/SIV infection, there is a significant and progressive insult to the intestinal mucosa, beginning with massive infection of and subsequent depletion of CD4+ T cells and eventual compromise of the structural epithelial barrier of the gastrointestinal (GI) tract.1, 2 These changes lead to translocation of GI tract microbial products,1, 3, 4 which contributes to persistent pathological immune activation and exacerbation of disease progression.3, 5, 6, 7, 8 However, the mechanisms responsible for the damage to the tight epithelial barrier of the GI tract during HIV/SIV infection, which may initiate these processes, remain unclear.
Another manifestation of mucosal immune dysfunction during pathogenic HIV/SIV infections is the preferential loss of interleukin (IL)-17-producing CD4+ and CD8+ T cells from mucosal tissues.9, 10, 11, 12, 13, 14, 15, 16 These IL-17-producing T cells are essential for mucosal immunity as they respond to extracellular bacteria and fungi, and are involved in maintenance of the structural barrier of the GI tract, driven by production of cytokines such as IL-22, as well as induction of claudin, antibacterial defensin, and/or mucin expression.10, 17, 18, 19, 20, 21 While T lymphocytes are clearly important sources of IL-17 and IL-22, recent studies have demonstrated that T cells are not the only lymphocytic source of IL-17-associated cytokines in vivo, nor are they the only subset important for maintenance of the structural barrier of the GI tract and mucosal immunity. Indeed, specialized subsets of innate lymphocytes in mucosal tissues can produce IL-22 and/or IL-17, and contribute to homeostasis of epithelial cells along mucosal surfaces.22, 23, 24, 25, 26, 27 Furthermore, it was recently demonstrated that NKp44+ natural killer (NK) cells produce IL-17 and are lost from mucosal sites in progressive SIV infection.16
With continuous exposure to dietary antigens and commensal microbes, the gut represents a unique immunological environment, requiring a balance between prompt, effective responses to pathogens and tolerance of frequent exposures to noninfectious antigens. Appropriate mucosal immune responses depend on specialized dendritic cells (DCs) to sample GI tract antigens, migrate to lymphoid tissues, and present antigens to the adaptive immune system to convey signals critical for directing lymphocytes to either become activated or to maintain tolerance against specific antigens.28, 29, 30, 31 One such specialized subset of antigen-presenting cells in the GI tract is CD103+ DCs, which can metabolize vitamin A into retinoic acid (RA).31, 32, 33 This is of particular importance as RA production is essential for inducing the expression of RORγt, a transcription factor required for development and maintenance of IL-17- and IL-22-producing cells.34, 35, 36, 37, 38, 39, 40, 41 Thus, interactions between different DC and lymphocyte populations in the mucosal immune system define the unique local immune microenvironment, and disruption of these cells and their interactions may contribute to the mucosal immune dysfunction seen during pathogenic HIV/SIV infections.
We studied the frequency, phenotypes, and functionality of mucosal IL-17- and IL-22-producing lymphocytes, comparing results for uninfected and SIV-infected rhesus macaques (RMs) to assess the potential contribution of loss or dysfunction of these cells to loss of GI tract structural integrity and associated aspects of disease progression. Given the close and critical interactions between mucosal antigen presenting cells (APCs) and lymphocytes in the mucosal immune system, we also studied the frequency, phenotype, and functionality of mucosal APCs as a potential factor contributing to the loss of IL-17-producing lymphocytes. Our results reveal that SIV infection results in multiple disruptions in these cell populations and their interactions, and provide novel insights and a plausible mechanistic basis for the mucosal immune system dysfunction and the GI epithelial damage observed in progressive HIV/SIV infections.
Results
Loss of IL-17-producing lymphocytes is associated with damage to the mucosal barrier of the colon
As recent studies have demonstrated the importance of IL-17 in mucosal immunity,42 we performed flow cytometric analysis of IL-17-producing lymphocytes, including CD4+ T cells (Th17), CD8+ T cells (Tc17), and CD20-CD3-CD8+ NK-like lymphocytes from mucosal tissues of uninfected and chronically SIV-infected RM, after mitogenic stimulation (Supplementary Figures 1, 2a online). Of note, while CD20-CD3-CD8+ lymphocytes in peripheral blood are classically defined as NK cells,43 in mucosal tissues these cells do not homogenously express any given NK cell marker, including NKp44, NKp46, NKp80, or NKG2A (data not shown), and thus are referred to as “CD3-CD8+ lymphocytes”. This ex vivo analysis showed all subsets of IL-17-producing lymphocytes were decreased in mesenteric lymph node (MLNs) and colon tissues of chronically SIV-infected RMs compared with uninfected RMs (Supplementary Figure 1a, b online). As the flow cytometry analysis of tissue-derived cells addresses only the relative frequencies of different populations, to discriminate between the relative loss of IL-17-producing lymphocytes and potential influx of non-IL-17+ cells in explaining this observation, we performed immunohistochemical analysis and quantitative analysis of IL-17+ cells in paraffin-embedded colonic tissue from SIV-infected and uninfected RMs to assess the absolute numbers of IL-17+ cells per unit area of lamina propria (Supplementary Figure 1c, d online). These data confirmed that IL-17-producing cells were in fact depleted from the GI tract of SIV-infected RMs with a significant loss of IL17+ cells per mm2 in the lamina propria of the colon (P=0.0012, Supplementary Figure 1e online). Preservation of IL-17-producing lymphocyte subsets in tissues of SIV-infected natural host sooty mangabeys (SMs; Supplementary Figure 1f online), which replicate SIV to high levels after SIV infection but do not develop GI mucosal damage and immunological dysfunction or progress to simian AIDS, is consistent with a potential role for these cells in maintenance of mucosal integrity in nonprogressive SIV infection.
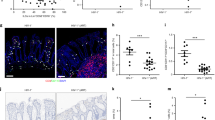
A hallmark of progressive HIV/SIV infection is focal damage to the epithelial barrier of the colon, leading to microbial translocation,1 but the mechanism(s) underlying this damage and associated changes in intestinal permeability are unknown. Given data implicating IL-17-producing lymphocytes in GI tract immunity and maintenance of GI epithelial integrity, we investigated the relationship between loss of different IL-17-producing lymphocyte populations and structural, multifocal, damage to the GI tract using immunohistochemical analysis with antibodies against the tight junction protein claudin-3 to assess the continuity of the structural epithelial barrier of the colon (breach/intact ratio) as previously described.1, 44 We found a significant, inverse correlation between the frequency of colonic Th17 cells and damage to the epithelial barrier of the colon (P=0.0219, r=−0.6643, Figure 1a), and a trend toward a negative relationship between MLNs Th17 cell frequency and structural damage (P=0.1351, r=−0.4198, Figure 1b). For Tc17 cells, we found a significant, inverse correlation between the extent of damage to the epithelial barrier of the colon and the frequency of Tc17 cells in both the colon (P=0.0033, r=−0.7902, Figure 1c) and MLNs (P=0.0389, r=−0.5560, Figure 1d). Intriguingly, we observed the most robust inverse correlations for the extent of colonic epithelial damage and the frequency of IL-17-producing CD3-CD8+ lymphocytes in the colon (P=0.0011, r=−0.7758, Figure 1e) and MLNs (P=0.0009, r=−0.7846, Figure 1f). These significant correlations were maintained when SIV-uninfected RM were excluded from the analysis (data not shown). Furthermore, we also found inverse correlations between the frequency of mucosal IL-17-producing lymphocytes and systemic immune activation, as measured by Ki67 expression by peripheral blood CD4+ and CD8+ T cells, suggesting that loss of mucosal IL-17-producing lymphocytes is not only associated with epithelial damage but also with the persistent, systemic immune activation that characterizes pathogenic HIV/SIV infection (data not shown). Given the role of IL-17-producing lymphocytes in contributing to the maintenance of the epithelial barrier of the GI tract, these data suggest that loss of these cells may provide an explanation for the observed damage to the tight epithelial barrier of the GI tract and the ensuing microbial translocation and pathological systemic immune activation characteristic of progressive SIV infection of RMs.
Loss of interleukin (IL)-17-producing lymphocytes correlates with damage to the tight epithelial barrier of the colon. The frequency of IL-17-producing CD4+ T cells in (a) colon and (b) mesenteric lymph nodes (MLNs), IL-17-producing CD8+ T cells in (c) colon and (d) MLNs, and IL-17-producing CD3-CD8+ lymphocytes in (e) colon and (f) MLNs were compared with the extent of damage to the structural barrier of the colonic epithelium as measured by the breach (no claudin) to intact (claudin) ratio. Circles, SIV−; Triangles, SIV+. Diagonal lines represent linear regression.
IL-17-producing lymphocytes express genes essential for enterocyte homeostasis
While IL-17 production is associated with preservation of epithelial cell homeostasis, the actual mechanism by which IL-17-producing lymphocytes exert this protective function is unclear. To further characterize these cells, we performed gene array analysis on messenger RNA isolated from MLN lymphocyte subpopulations separated based upon production of IL-17 following mitogenic stimulation. Supervised cluster analysis of results for cells that did or did not produce IL-17 demonstrated distinct gene expression profiles, apparent in heatmap cluster analysis of the top 200 differentially expressed genes (Figure 2a). Further analysis of genes differentially expressed between the two subsets revealed that the gene most significantly upregulated in the IL-17+ lymphocytes was IL-17F, validating our approach for separating IL-17-producing and nonproducing lymphocytes (Figure 2b). Bioinformatic analysis identified more than 1,269 genes that were significantly differentially regulated between the two subsets (fold change ±1.5 and P<0.05). Furthermore, analysis of specific genes demonstrated several key functional distinctions between IL-17-producing and non-IL-17-producing lymphocytes based on false discovery rate-adjusted P values for the IL-17− vs. IL-17+ microarray-specific genes calculated using the R package Limma (Bioconductor: http://www.bioconductor.org). Several genes that are essential for GI tract function were upregulated in IL-17+ lymphocytes, including IL-22, which is an essential growth factor for epithelial cells27, 45 (Figure 2b). In addition, the chemokines CCL1 and CCL20, both of which are immune chemoattractants important for mucosal immunity, were upregulated in IL-17+ lymphocytes.46, 47 Furthermore, IL-17+ lymphocytes also expressed the integrin β7, which is essential for mucosal homing and retention of lymphocytes,48 and TNFRSF4 (OX40), a co-stimulatory molecule of the tumor necrosis factor super family implicated in regulation and survival of mucosal lymphocytes,49, 50 as well as RORc, which encodes RORγt, and is essential for IL-17 production.51, 52
Interleukin (IL)-17-producing lymphocytes upregulate genes essential for mucosal homeostasis. (a) The top 200 genes based on P value for the greatest contrast between IL17+ and IL17− lymphocytes from mesenteric lymph nodes of SIV-uninfected rhesus macaque (RM). The complete linkage method was used for hierarchical cluster analysis. Both rows and columns are clustered. Each row represents normalized expression value for a single gene, and each column represents a sample (data are based upon RNA extractions from IL17+ lymphocytes from two SIV-uninfected RM, left, and IL17− lymphocytes from three SIV-uninfected RM, right). (b) Fold change of selected genes that were upregulated by IL-17+ lymphocytes (top) or IL-17− lymphocytes (bottom). P values donated above each gene fold change. (c–d) The network (c) and pathway (d) from Ingenuity Pathway Analysis for selected genes is shown. Red indicates upregulation (fold change >=1.5); green indicates downregulation (fold change <=−1.5); grey indicates genes whose expression values are between −1.5 and 1.5. (c) Shape: triangle represents kinase; square represents cytokine; rectangle represents ligand-dependent nuclear receptor; diamond represents enzyme; trapezoid represents transporter; ellipse represents transcription regulator; circle represents others. HMG, hydroxy methyl glutaryl(-CoA synthase); BCG, bacille Calmette-Guerin (vaccine); IFN, interferon; TGF, transforming growth factor.
In comparison, in the IL-17− lymphocytes, genes essential for Th1 and Th2 responses were notably upregulated, including genes associated with interferon signaling and production, by IFNGR1, STAT3, and STAT6.53 Furthermore, genes associated with transforming growth factor-β signaling, including TGIF1,54 were also expressed by the IL-17− lymphocytes (Figure 2b). A complete list of these genes is presented (Supplementary Table 1).
Network analysis of the expression profile of the IL-17+ lymphocytes (compared with IL-17−) demonstrated the association of genes essential for promotion of IL-17 and IL-22 in these mucosal lymphocytes (Figure 2c). T-helper cell pathway analysis further demonstrates the skewing of the expression profile of the IL-17+ cells away from a “Th1” phenotype, and toward a “Th17” phenotype (Figure 2d). Thus, the transcriptional analysis of IL-17-producing lymphocytes demonstrated expression of genes implicated in mucosal immunity and maintenance of the GI tract epithelial barrier.
IL-22-producing lymphocytes are lost after SIV infection in association with damage to the GI tract epithelial structural barrier
Recent studies have identified IL-22 as a cytokine important for enterocyte homeostasis.23, 25, 27, 45 Notably, our transcriptional analysis demonstrated that mucosal lymphocytes that express IL-17 upon stimulation express significantly higher levels of IL-22 transcript than IL-17− lymphocytes (4.53 fold change and P<0.0001) (Figure 2). To identify other cell types capable of producing IL-22 upon ex vivo stimulation, we used flow cytometry (Supplementary Figure 2b online). High frequencies of all subsets of IL-17+ lymphocytes from colon and MLNs also produced IL-22 after mitogenic stimulation (Figure 3a-b), findings consistent with our gene expression array data. Given this evidence of production of IL-22 by IL-17+ lymphocytes and the observation that IL-17+ lymphocytes are lost from mucosal tissues in SIV-infected RMs, we compared IL-22 production by mucosal CD4+ and CD8+ T cells and CD3-CD8+ lymphocytes from uninfected and SIV-infected RMs. In SIV-infected RMs, we found significantly decreased IL-22 production by CD4+ T cells from both colon and MLNs (P=0.0159, P=0.0048, Figure 3c and d, left), as well as from CD3−CD8+ lymphocytes in colon and MLNs (P=0.0491, P=0.0360, Figure 3c–d, right). We also observed a trend for decreased IL-22 production by CD8+ T cells from colon and MLNs of SIV-infected RMs (Figure 3c–d, center). Of note, production of IL-22 and IL-17 are not mutually exclusive; IL-22+IL-17+ as well as IL-22+IL-17− and IL-22-IL-17+ lymphocyte populations can all be demonstrated and are all decreased after SIV infection (data not shown). As IL-22 is known to be essential for enterocyte proliferation and homeostasis,45 we hypothesized that loss of IL-22 after SIV infection might also be associated with damage to the tight epithelial barrier of the GI tract. Indeed, examining different IL-22-producing lymphocyte subpopulations, we found significant inverse correlations between the frequency of colonic and MLNs CD4+IL-22+ T cells and damage to the structural barrier of the colon (P=0.0108, r=−0.8167; P=0.0068, r=−0.6760, Figure 3e–f, left), and inverse trends between the frequency of colon and MLNs CD8+IL-22+ T cells and damage to the epithelial barrier of the colon (Figure 3e–f, center). We also observed an inverse trend for the relationship between IL-22+CD3-CD8+ lymphocytes in the colon and damage to the barrier (P=0.0968, r=−0.6025, Figure 3e, right), and a significant inverse correlation in MLNs (P=0.0042, r=−0.6929, Figure 3f, right). Taken together, these data demonstrate that loss of IL-22-producing lymphocytes after SIV infection is associated with damage to the tight epithelial barrier of the GI tract.
Interleukin (IL)-17-producing lymphocytes produce IL-22, and IL-22-producing lymphocytes are lost after SIV infection and associated with mucosal barrier integrity. (a, b) The frequency of IL-22+ cells within IL-17-producing CD4+ T cells (left), CD8+ T cells (center), and CD3-CD8+ lymphocytes (right) in (a) colon and (b) mesenteric lymph nodes (MLNs). (c, d) The frequency of IL-22-producing CD4+ T cells (left), CD8+ T cells (center), and CD3-CD8+ lymphocytes (right) in uninfected (circles) or SIV-infected (triangles) RMs in (c) colon and (d) MLNs. (e, f) The frequency of IL-22-producing CD4+ T cells (left), CD8+ T cells (center), and CD3-CD8+ lymphocytes (right) in (e) colon and (f) MLNs were compared with damage to the structural barrier of the colon as measured by the breach (no claudin) to intact (claudin) ratio. Closed circles, SIV− colon; closed triangles, SIV+ colon; open circles, SIV− MLNs; open triangles, SIV+ MLNs. Horizontal bars represent median and diagonal lines represent linear regression.
CD103+ DCs are lost after SIV infection, and are associated with loss of IL-17- and IL-22-producing lymphocytes
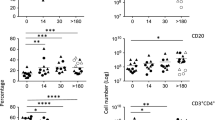
Given the critical importance for local APCs in priming lymphocytes toward specific functionalities and in maintaining cells with such functions, we investigated the relationship between the frequency of IL-17- and IL-22-producing lymphocytes and individual subsets of DCs within the GI tract and MLNs. We measured the frequencies of CD103+ DCs, identified as live, lineage-, HLA-DR+CD103+ cells, during SIV infection (Supplementary Figure 2c online) and found significant decreases in CD103+ DCs in both colon (P=0.0260, Figure 4a) and MLNs (P=0.0040, Figure 4b) in SIV-infected animals. To determine whether loss of these CD103+ DCs might contribute to the loss of IL-17- and IL-22-producing lymphocytes, we investigated the relationship between these cell subsets in colon and MLNs. We found a significant, positive correlation between the frequency of CD103+ DCs and Th17 cells (P<0.0001, r=0.7158, Figure 4c), Tc17 cells (P=0.0011, r=0.6249, Figure 4d), and IL-17+CD3-CD8+ lymphocytes (P=0.0053, r=0.5401, Figure 4e) in these mucosal tissues. Similarly, we found a significant, positive correlation when we compared the frequency of CD103+ DCs and CD4+IL-22+ T cells (P=0.0032, r=0.5876, Figure 4f), and IL-22+CD3-CD8+ lymphocytes (P=0.0317, r=0.4488, Figure 4h) and a trend toward an association between CD8+IL-22+ T cells and CD103+ DCs (Figure 4g). Of note, we found no significant changes in the frequencies of either CD11c+ myeloid DCs (Supplementary Figure 4a online) or CD123+ plasmacytoid DCs (Supplementary Figure 4b online) in the colon after SIV infection. Furthermore, we found no similar correlations between frequencies of either myeloid DCs (Supplementary Figure 4c online) or plasmacytoid DCs (Supplementary Figure 4d online) and IL-17+ lymphocytes in mucosal tissues. In addition, maintenance of CD103+ DCs during nonprogressive SIV infection of natural host SMs suggests conservation of CD103+ DCs in mucosal tissues of SMs may contribute to maintenance of healthy mucosal immunity during nonprogressive SIV infection (Supplementary Figure 4e online).
CD103+ dendritic cells (DCs) are lost after SIV infection, and associated with the frequency of IL-17- and IL-22-producing lymphocytes. (a, b) The frequency of live, lineage-, HLA-DR+ CD103+ dendritic cells (DCs) in the colon (a) or mesenteric lymph nodes (MLNs) (b). (c–e) The frequency of CD103+ DCs is compared with the frequency of IL-17-producing CD4+ T cells (c), CD8+ T cells (d), or CD3-CD8+ lymphocytes (e). (f–h) The frequency of CD103+ DCs is compared with the frequency of IL-22-producing CD4+ T cells (f), CD8+ T cells (g), or CD3-CD8+ lymphocytes (h). Closed circles, SIV− colon; closed triangles, SIV+ colon; open circles, SIV− MLNs; open triangles, SIV+ MLNs. Horizontal bars represent median and diagonal lines represent linear regression.
CD103+ DCs promote IL-17 and IL-22-producing lymphocytes
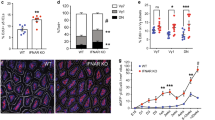
To characterize further CD103+ DCs and to understand whether these APCs can directly support development or maintenance of IL-17- and IL-22-producing lymphocytes, we performed transcriptional analysis of CD103+ and CD11c+CD103− DCs isolated from MLNs of uninfected macaques. Striking differences between the transcriptional profiles of these DCs were evident by heatmap-unsupervised cluster analysis of the top 200 differentially regulated genes (Figure 5a). In fact, we observed 1,177 genes that are significantly and differentially expressed (fold change ±1.5 and P value <0.05) when we compared CD103+ to CD103− DCs (a complete list of genes available in Supplementary Table 2 online). Furthermore, analysis of key differentially regulated genes demonstrated the induction of genes that strongly associated with the described functions of these DC subsets (Figure 5b). For example, CD103+ DCs expressed genes that are essential for the promotion of IL-17+ and IL-22+ lymphocytes, including the transcriptional regulator IL-6, along with MAP3K7, APOE, and RXRB (retinoid X receptor B) (Figure 5b). Furthermore, other immunomodulatory genes, such as CCL22, IL-15, and IRF3, were expressed by CD103+ DCs, further demonstrating the important role for CD103+ DCs in mucosal immune regulation (Figure 5b). In contrast, CD103-CD11c+ DCs expressed numerous genes essential for induction and regulation of interferon responses, including JAK1, IFI6, IRF8, and CXCL10 (Figure 5b), as well as immunomodulatory genes such as CCR4 and TGFB1 (Figure 5b). We performed gene network analysis to investigate potential associations of genes within the IL-17 pathways in CD103+ and CD103− DCs (Figure 5c). Increased expression of IL-6 represented a significant node within the network connecting IL-17, IL-22, and RXRA genes, all known to be important in the regulation and promotion of IL-17 (Figure 5c).
CD103+ dendritic cells (DCs) upregulate IL-17-promoting genes. (a) The top 200 genes based on P value for the contrast between CD103+DCs and CD103-CD11c+ DCs from mesenteric lymph nodes (MLNs) of SIV-uninfected rhesus macaque. The complete linkage method was used for hierarchical cluster analysis. Both rows and columns are clustered. Each row represents normalized expression value for a single gene, and each column represents a sample (data are from MLNs of three SIV-uninfected animals, and CD103+DCs are left and CD103-CD11c+ DCs are right). (b) Fold change of selected genes that were upregulated in CD103+ DCs (top) or CD103-CD11c+ DCs (bottom). P values donated above each gene fold change. (c) The network from Ingenuity Pathway Analysis for selected genes is shown. Color: red indicates upregulation (fold change >=1.5); green indicates downregulation (fold change <=−1.5); grey indicates the genes whose expression values are between −1.5 and 1.5. Shape: triangle represents kinase; square represents cytokine; rectangle represents ligand-dependent nuclear receptor; diamond represents enzyme; trapezoid represents transporter; ellipse represents transcription regulator; and circle represents others.
CD103+ DCs can directly promote a Th17 phenotype in vitro
While transcriptional profiling demonstrated gene expression associated with IL-17 and IL-22 production, to address whether mucosal CD103+ DCs can directly prime naïve CD4+ T cells toward a Th17 phenotype, we performed cocultures with flow cytometrically sorted, naïve CD4+ T cells and DCs isolated from the MLNs of two SIV-uninfected RMs. Naïve CD4+ T cells were cultured with either CD103+ DCs, CD103− DCs, or no DCs, and anti-CD3/anti-CD28 beads (control) under Th17 conditions, with or without IL-6. We found that in the presence of IL-6, CD103+ DCs promoted IL17A expression that was 1.88-fold higher than expression by CD4+ T cells cultured with CD103− DCs (Figure 6a). Furthermore, the importance of IL-6 in promoting IL-17 production was demonstrated, as cocultures with CD103+ DCs in the absence of IL-6 expressed 0.84-fold less IL17A (Figure 6b), though CD4+ T cells cocultured with CD103+ DCs maintained higher expression of IL17A (Figure 6b), suggesting that other functions of CD103+ DCs, such as RA production, are also essential for the promotion of IL17 production. In addition to IL17A, we also measured the expression of RORc, which encodes RORγt, the transcription factor essential for IL-17 and IL-22 production41 (Figure 6c–d). We found that CD4+ T cells cocultured with CD103+ DCs expressed, on average, 6.31-fold higher levels of RORc compared with CD4+ T cells cocultured with CD103− DCs (Figure 6c). CD103+ DC cocultures without IL-6 expressed an average of 3.27-fold less RORc than cultures with IL-6, however, still expressed levels of RORc higher than cocultures with CD103− DCs (Figure 6d). Of note, owing to the very low numbers of CD103+ APCs obtainable from MLNs and the very large numbers of cells required for these experiments, we could only isolate a sufficient number of cells from two sacrificed RMs (Rh1 and Rh2), thus statistical analysis was not possible, though the same trends were present in all animals in all conditions. Consistent with this implied role for RA in inducing Th17 cells in vivo, in a separate pilot experiment, we found moderate increases in the frequencies of Th17 cells in a chronically SIV-infected animal therapeutically dosed with 50 mg kg−1 all trans-RA subcutaneously (data not shown).
CD103+ dendritic cells (DCs) directly promote IL17A and RORc expression by naïve CD4+ T cells. Naïve CD4+ T cells were cocultured under Th17 conditions with stimulatory anti-CD3 beads alone (control), with CD103+ DCs, or with CD103−CD11c+ DCs in the presence of interleukin (IL)-6 (a, c) or absence of IL-6 (b, d). Gene expression for IL17A (a, b) and RORc (c, d) was measured by ΔΔCt compared with controls. Cells were sorted from live, SIV− rhesus macaque (RM) mesenteric lymph nodes. Rh1 and Rh2 designate the two RMs used for this experiment.
Taken together, our data demonstrate that CD103+ DCs can directly induce naïve T cells to express IL17A and RORc. In contrast, other DCs can produce indoleamine 2,3-dioxygenase (IDO), which has been shown to be inversely associated with frequencies of Th17 cells during pathogenic SIV and HIV infections.13, 16, 55 Indeed, we found that increased IDO expression in mucosal tissues (Supplementary Figure 5a—f online) significantly, negatively correlates with the frequencies of CD103+ DCs (Supplementary Figure 5g–h online). Thus, alterations in the mucosal APC landscape after SIV infection likely contribute to the decreased CD103+ DCs and IL-17+ and IL-22+ lymphocytes we observe here and provide a plausible mechanism whereby maintenance of mucosal CD103+ DCs promoting the development and survival of IL-17+ and IL-22+ lymphocytes is important for maintenance of the tight epithelial barrier of the GI tract and mucosal immunity.
Discussion
To understand the potential mechanisms by which SIV infection leads to focal damage to the intestinal epithelial barrier, resulting in microbial translocation that contributes to persistent immune activation, we studied subpopulations of resident immune cells in the GI tract and draining MLNs, comparing uninfected and chronically SIV-infected RMs, as well as nonprogressively SIV-infected and uninfected SMs. We have shown that CD4+ T cells, CD8+ T cells, and CD3-CD8+ lymphocytes isolated from mucosal tissues can produce IL-17 and IL-22 after mitogenic stimulation, and were significantly decreased after pathogenic SIV infection. Furthermore, transcriptional analysis revealed multiple genes expressed by IL-17-producing lymphocytes that are essential for enterocyte homeostasis and mucosal immune function. Indeed, IL-22, which is essential for enterocyte proliferation, was upregulated by IL-17+ cells, and we show that IL-22 is produced by IL-17+ cells and these lymphocyte populations are lost in pathogenic SIV infection. Importantly, loss of these IL-17- and IL-22-producing lymphocytes was associated with damage to the tight epithelial barrier of the GI tract. This suggests that IL-17- and IL-22-producing lymphocytes may have an essential role in the maintenance of the GI tract epithelial barrier, and that loss of these cells during HIV/SIV infections may contribute to the damage observed.
As we found no correlations between levels of plasma viral load and frequencies of IL-17- or IL-22-producing lymphocytes (data not shown), viral replication, itself, is unlikely leading to direct loss of these cells. Indeed, previous studies found no evidence for preferential infection of Th17 cells, and neither CD3-CD8+ lymphocytes nor CD8+ T cells capable of expressing IL-17 are targets for the virus in vivo.9 Furthermore, preservation of IL-17-producing lymphocytes in natural hosts, which maintain GI epithelial integrity and avoid microbial translocation, persistent systemic immune activation, and progressive disease despite high viral loads during infection, indicates that virus replication is not sufficient for the dysfunction of the mucosal immune system observed in progressively SIV-infected RM.
Our results suggest that modulation of different mucosal APC populations represents a plausible mechanism underlying loss of mucosal IL-17- and IL-22-producing cells. Increased activation and inflammation in mucosal tissues after SIV infection may lead to increased levels of proinflammatory immune cells that may inhibit CD103+ DCs through mechanisms such as tryptophan metabolism manifested by upregulated IDO. Furthermore, the general activated state in the mucosa may promote the influx of extrinsic DC populations that do not, generally, reside within mucosal tissues, thus overwhelming the numbers and/or effects of mucosal-resident CD103+ DCs. In turn, lack of CD103+ DCs in the mucosal tissues likely results in decreased IL-6 production and metabolism of vitamin A to RA, and the combination of decreased IL-6 and RA and increased IDO could easily lead to loss of IL-17-producing lymphocytes. Indeed, we have demonstrated here that CD103+ DCs have increased expression of IL-6 genes compared with CD103− DCs, and that removing IL-6 from cocultures results in decreased IL17A and RORc expression. This loss of the IL-17- and IL-22-producing cells may contribute to damage to the tight epithelial barrier of the GI tract with subsequent microbial translocation and immune activation; all of which contribute to disease progression during pathogenic SIV and HIV infection. Of note, previous studies in mice have shown that CD103+ DCs specialize in promoting T-regulatory cells;32, 33 however, recent murine studies have demonstrated that, consistent with our primate results, RA and IL-6 produced by CD103+ DCs are also essential for IL-17 production.36 Furthermore, recent studies have demonstrated that the functional specializations of intestinal DCs in mice is dependent on many factors, including source of mice and APC:T cell ratios,56 demonstrating the diverse functionality and importance of CD103+ DCs.
In conclusion, here we have demonstrated that multiple IL-17- and IL-22-producing lymphocyte subsets are preferentially lost from mucosal tissues of chronically SIV-infected RM, and that this loss may adversely affect GI tract epithelial homeostasis. Furthermore, loss of IL-17-producing cells is associated with loss of CD103+ DCs, which we show can directly promote IL17A as well as RORc, essential for induction of IL-17 and IL-22 production by lymphocytes. Furthermore, transcriptional analysis demonstrates that CD103+ DCs express genes essential for survival of mucosal IL-17+ and IL-22+ lymphocytes. These data provide mechanistic insight underlying dysregulation of mucosal immunity during HIV/SIV infections, and may provide clues for developing therapeutic strategies intended to preserve or restore these populations, potentially improving the epithelial integrity of the GI tract and limiting microbial translocation and the associated immune activation.
Methods
Animals and sample collection. For this study, 12 chronically (day 90+) SIV-infected RMs (Macaca mulatta) and 6 SIV-uninfected RMs were euthanized, and blood, mesenteric LN, and colon tissues were collected. Of the 12 chronically SIV-infected RMs, 3 animals were infected intravenously with 1 TCID50, 3 with 10 TCID50, and 3 intrarectally with 3,000 TCID50 of SIVsmE543, and 3 animals were infected intravenously with 3,000 TCID50 of SIVmac239. RMs were infected with multiple pathogenic viruses and via different inoculation routes, and animals were killed at different time points post SIV infection in order to establish a diverse cohort of progressively SIV-infected animals, the goal being to ensure our observations were reproducible in multiple infection scenarios and to maximize our chances of observing a spectrum of immunological abnormalities (Supplementary Table 3 online). None of the animals had clinical signs of simian AIDS. Ten rectal pinch biopsies and blood samples were also collected from 19 naturally SIV-infected SM (Cercocebus atys) during chronic infection and from 15 SIV-uninfected SMs. Lymphocytes were isolated as previously described.44 Animals were housed and cared in accordance with the American Association for Accreditation of Laboratory Animal Care standards in AAALAC-accredited facilities, and all animal procedures were performed according to the protocols approved by the Institutional Animal Care and Use Committees of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (RMs), or of Emory University (SMs).
Flow cytometry. Multicolor flow cytometric analysis was performed on samples stimulated and stained as previously described44 using cross-reactive antihuman antibodies: CD3 (clone SP34-2, BDPharmigen, Franklin Lakes, NJ), CD8 (RPA-T8, BDPharmigen), CD8 (3B5, Invitrogen, Carlsbad, CA), CD4 (OKT4, eBioscience, San Diego, CA), Ki67 (B26, BDPharmigen), HLA-DR (L243, BDPharmigen), CD45 (MB46D6, Miltenyi, Auburn, CA), CD14 (M5E2, BDPharmigen or TUK4, Invitrogen), CD20 (2H7, eBioscience), interferon-γ (IFN-γ) (4S.B3, BDPharmigen), IL-17 (eBio64CAP17, eBioscience), CD103 (B-Ly7, eBioscience), IL-22 (IL22JOP, eBioscience), CD123 (6H6, eBioscience), CD11c (3.9, eBiocience), and Aqua Live/Dead amine dye (Invitrogen). Flow cytometric acquisition was performed on a BD (Franklin Lakes, NJ) LSRFortessa driven by the FACS DiVa software (v6.0; BD). Analysis of the acquired data was performed using FlowJo software (v9.0.2; TreeStar, Ashland, OR). We used a minimum threshold of 200 collected events for all analysis. We performed Mann–Whitney U-test for all t-test P values, horizontal bars in figures reflect median. Spearman rank calculation and linear regression were calculated for comparative correlations, performed using Prism 5.0 software (Graphpad Prism, La Jolla, CA).
Immunohistochemistry and quantative image analysis. Immunohistochemical staining was performed as previously described1, 44 on specimens of tissues fixed in Prefer fixative (Anatech, Battle Creek, MI) or neutral-buffered 4% paraformaldahyde (Electron Microscopy Sciences, Hatfield, PA). Primary antibodies used were polyclonal rabbit anti-claudin-3 (Labvision, Kalamazoo, MI), polyclonal rabbit anti-IDO (Millipore, Billerica, MA), and monoclonal human anti-IL-17 (clone eBio64CAP17, eBioscience). We performed Mann–Whitney U-test for all t-test P values, horizontal bars in figures reflect median. Spearman rank calculation and linear regression were calculated for comparative correlations, performed using Prism 5.0 software (Prism).
DC Cocultures. Sorted naïve (live, singlet, CD3+CD28+CD95-CCR7+CD27+) CD4+ T cells were cocultured with sorted CD103+ DCs (live, lineage-CD14-HLA-DR+CD11c+) (100:1), sorted CD103− DCs (lineage-CD14-HLA-DR+CD11c+CD103-) (100:1), or anti-CD3/anti-CD28 beads (control, 4:1) in X-Vivo15 media (Lonza, Walkersville, MD) under Th17 conditions (10 U ml−1 recombinant human IL-2, 12.5 ng ml−1 rhIL-1β, 25 ng ml−1 rhIL-21, 25 ng ml−1 rhIL-23, 10 μg ml−1 anti-IFN-γ, 10 μg ml−1 anti-IL-12, and 2 ng ml−1 transforming growth factor-β, with or without 25 ng ml−1 rhIL-6). All cytokines were from R&D (Minneapolis, MN), except IL-2, IL-6, and IL-21 (Peprotech, Rocky Hill, NJ). DC cocultures were stimulated with Staphylococcal enterotoxin B (SEB) (1 μg ml−1, Sigma Aldrich, St Louis, MO). All cultures were fed on day 3 with rhIL-2 (2 U ml−1), anti-IFN-γ (10 μg ml−1), and anti-IL-12 (10 μg ml−1). After 7 days of coculture, quantitative real-time PCR was performed for IL17A and RORc gene expression (Applied Biosystems, Carlsbad, CA). Gene expression was quantified using ΔΔCT analysis in excel (version 12.3.0, Microsoft Excel, Redmond, WA).
Microarray analysis. For microarray analysis, four subsets of live cells were sorted from SIV− RM MLNs: CD45+ IL-17+ lymphocytes, as measured using an IL-17 secretion/enrichment and detection assay (Miltenyi) after 4 h of phorbol 12-myristate 13-acetate and ionomycin stimulation, and CD45+ lymphocytes that were IL-17-, as well as live, lineage-, HLA-DR+ CD103+ DCs, and CD103-CD11c+ DCs. Eleven RNA samples (four groups: CD103+CD11c- DCs, CD103-CD11c+ DCs, IL17+ MLNs lymphocytes, and IL17-MLNs lymphocytes) were hybridized to the Illumina (San Diego, CA) HumanHT-12 version 4 Expression BeadChip according to the manufacturer's instructions, and quantified using an Illumina iScan System. The data were collected with Illumina GenomeStudio software, normalized with the quantile normalization method of the Bioconductor package limma,57 and then log-transformed. Missing values were imputed with R package impute (http://cran.r-project.org/web/packages/impute/index.html). Two contrasts were analyzed from MLNs of SIV-uninfected animals; CD103+CD11c- DCs vs. CD103-CD11c+ DCs, and IL17+ vs. IL17− lymphocytes. Genes with significant differential expression levels were identified using Bioconductor limma package with ≥1.5 fold change (up or down), and the raw P value <0.05. P values are corrected for multiple comparisons. We used the R package Limma (http://bioconductor.org/packages/release/bioc/html/limma.html) to estimate the false discovery rate-adjusted P values, and as a parametric t-test is inappropriate for our small sample size with normal distribution, we used the linear model with empirical Bayes method;57 these analyses are stable even for experiments with small number of arrays (http://www.statsci.org/smyth/pubs/limma-biocbook-reprint.pdf, page 397).
Ingenuity pathway analysis. All network analysis was done with Ingenuity Pathway Analysis (IPA: Ingenuity Systems, Redwood City, CA). The input data include genes whose expression levels meet the following criteria: fold change >=1.5 (up or down) and raw P value < 0.05. Genes were mapped to the Ingenuity pathway knowledge base with different colors (red: upregulated; green: downregulated) based on Entreze Gene IDs. The canonical pathways were ranked with –log P values. The network for a few selected genes, including IL17F, IL22, and IRF3, was further explored based on the Ingenuity knowledge base.
References
Estes, J.D. et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 6, e1001052 (2010).
Brenchley, J.M. & Douek, D.C. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 1, 23–30 (2008).
Brenchley, J.M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371 (2006).
Brenchley, J.M., Price, D.A. & Douek, D.C. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7, 235–239 (2006).
Ancuta, P. et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE 3, e2516 (2008).
Marchetti, G. et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS 22, 2035–2038 (2008).
Baroncelli, S. et al. Microbial translocation is associated with residual viral replication in HAART-treated HIV+ subjects with <50copies/ml HIV-1 RNA. J. Clin. Virol. 46, 367–370 (2009).
Jiang, W. et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J. Infect. Dis. 199, 1177–1185 (2009).
Brenchley, J.M. et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112, 2826–2835 (2008).
Cecchinato, V. et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 1, 279–288 (2008).
Lederer, S. et al. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 5, e1000296 (2009).
Paiardini, M. Th17 cells in natural SIV hosts. Curr. Opin. HIV AIDS 5, 166–172 (2010).
Favre, D. et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 5, e1000295 (2009).
Nigam, P., Kwa, S., Velu, V. & Amara, R.R. Loss of IL-17-producing CD8T cells during late chronic stage of pathogenic SIV infection. J. Immunol. (2010).
Gordon, S.N. et al. Disruption of intestinal CD4+ T cell homeostasis is a key marker of systemic CD4+ T cell activation in HIV-infected individuals. J. Immunol. 185, 5169–5179 (2010).
Reeves, R.K. et al. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood 118, 3321–3330 (2011).
Huang, W., Na, L., Fidel, P.L. & Schwarzenberger, P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 190, 624–631 (2004).
Chung, D.R. et al. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J. Immunol. 170, 1958–1963 (2003).
Brand, S. et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G827–G838 (2006).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Chen, Y. et al. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 278, 17036–17043 (2003).
Crellin, N.K., Trifari, S., Kaplan, C.D., Cupedo, T. & Spits, H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J. Exp. Med. 207, 281–290 (2010).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Cella, M., Otero, K. & Colonna, M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc. Natl Acad. Sci. USA 107, 10961–10966 (2010).
Spits, H. & Di Santo, J.P. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 12, 21–27 (2010).
Lochner, M. et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J. Exp. Med. 208, 125–134 (2010).
Malmberg, K.J. & Ljunggren, H.G. Spotlight on IL-22-producing NK cell receptor-expressing mucosal lymphocytes. Nat. Immunol. 10, 11–12 (2009).
Simon, M., Ulf, Y., Vuk, C. & Gordon, M. Subsets of migrating intestinal dendritic cells. Immunol. Rev. 234, 259–267 (2010).
King, I.L., Kroenke, M.A. & Segal, B.M. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J. Exp. Med. 207, 953–961 (2010).
Milling, S., Yrlid, U., Cerovic, V. & MacPherson, G. Subsets of migrating intestinal dendritic cells. Immunol. Rev. 234, 259–267 (2010).
Schulz, O. et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 206, 3101–3114 (2009).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Jaensson, E. et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 205, 2139–2149 (2008).
Annunziato, F., Cosmi, L., Liotta, F., Maggi, E. & Romagnani, S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int. Immunol. 20, 1361–1368 (2008).
Cha, H.R. et al. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J. Immunol. 184, 6799–6806 (2010).
Hall, J.A. et al. Essential role for retinoic acid in the promotion of CD4+ T Cell effector responses via retinoic acid receptor alpha. Immunity 34, 435–447 (2011).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Svensson, M. et al. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 1, 38–48 (2010).
Wang, C., Kang, S.G., HogenEsch, H., Love, P.E. & Kim, C.H. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J. Immunol. 184, 5519–5526 (2010).
Jaensson-Gyllenback, E. et al. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 4, 438–447 (2010).
Sawa, S. et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science 330, 665–669 (2010).
Klatt, N.R. & Brenchley, J.M. Th17 cell dynamics in HIV infection. Curr. Opin. HIV AIDS 5, 135–140 (2010).
Reeves, R.K. et al. CD16- natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood 115, 4439–4446 (2010).
Klatt, N.R. et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 3, 387–398 (2010).
Sanos, S.L., Vonarbourg, C., Mortha, A. & Diefenbach, A. Control of epithelial cell function by interleukin-22-producing RORgammat+ innate lymphoid cells. Immunology 132, 453–465 (2011).
Haque, N.S., Fallon, J.T., Taubman, M.B. & Harpel, P.C. The chemokine receptor CCR8 mediates human endothelial cell chemotaxis induced by I-309 and Kaposi sarcoma herpesvirus-encoded vMIP-I and by lipoprotein(a)-stimulated endothelial cell conditioned medium. Blood 97, 39–45 (2001).
Williams, I.R. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann. NY Acad. Sci. 1072, 52–61 (2006).
Shaw, S.K. & Brenner, M.B. The beta 7 integrins in mucosal homing and retention. Semin. Immunol. 7, 335–342 (1995).
Griseri, T., Asquith, M., Thompson, C. & Powrie, F. OX40 is required for regulatory T cell-mediated control of colitis. J. Exp. Med. 207, 699–709 (2010).
Withers, D.R. et al. The survival of memory CD4+ T cells within the gut lamina propria requires OX40 and CD30 signals. J. Immunol. 183, 5079–5084 (2009).
Ivanov, II et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Annunziato, F. et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204, 1849–1861 (2007).
Leonard, W.J. Role of Jak kinases and STATs in cytokine signal transduction. Int. J. Hematol. 73, 271–277 (2001).
Pessah, M. et al. c-Jun interacts with the corepressor TG-interacting factor (TGIF) to suppress Smad2 transcriptional activity. Proc. Natl Acad. Sci. USA 98, 6198–6203 (2001).
Baban, B. et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J. Immunol. 183, 2475–2483 (2009).
Denning, T.L. et al. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J. Immunol. 187, 733–747 (2011).
Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article 3 (2004).
Acknowledgements
We would like to acknowledge Heather Cronise, JoAnne Swerczek, Richard Herbert, and all the veterinary staff at the NIH animal center. We would like to thank Tracy Meeker and Stephanie Ehnert, and the veterinary staff at YNPRC. We would like to thank the Cleveland Immunopathogenesis Consortium (BBC/CLIC) for advice and helpful discussions. We would like to thank Mark Cameron and Peter Wilkinson from the Genomics core at VGTI-FL for gene array consultation. Histology support was provided by the Pathology/Histotechnology Laboratory (PHL) core service located at the National Cancer Institute-Frederick, Frederick, MD, USA. These studies were supported by the Intramural National Institute of Allergy and Infectious Diseases, US National Institutes of Health program, and in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E, and National Institutes of Health R01 AI-084836 under M Paiardini. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
About this article
Cite this article
Klatt, N., Estes, J., Sun, X. et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol 5, 646–657 (2012). https://doi.org/10.1038/mi.2012.38
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2012.38
This article is cited by
-
Immunopathogenesis in HIV-associated pediatric tuberculosis
Pediatric Research (2022)
-
Innate lymphoid cell dysfunction during long-term suppressive antiretroviral therapy in an African cohort
BMC Immunology (2021)
-
Innate Lymphoid Cells: Their Contributions to Gastrointestinal Tissue Homeostasis and HIV/SIV Disease Pathology
Current HIV/AIDS Reports (2019)
-
HIV and the Gut Microbiota: Composition, Consequences, and Avenues for Amelioration
Current HIV/AIDS Reports (2019)
-
IL-17 and IL-22 production in HIV+ individuals with latent and active tuberculosis
BMC Infectious Diseases (2018)